Cell-derived extracellular vesicles and membranes for tissue repair
- PMID: 34789267
- PMCID: PMC8600774
- DOI: 10.1186/s12951-021-01113-x
Cell-derived extracellular vesicles and membranes for tissue repair
Abstract
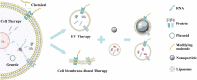
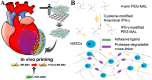
Humans have a limited postinjury regenerative ability. Therefore, cell-derived biomaterials have long been utilized for tissue repair. Cells with multipotent differentiation potential, such as stem cells, have been administered to patients for the treatment of various diseases. Researchers expected that these cells would mediate tissue repair and regeneration through their multipotency. However, increasing evidence has suggested that in most stem cell therapies, the paracrine effect but not cell differentiation or regeneration is the major driving force of tissue repair. Additionally, ethical and safety problems have limited the application of stem cell therapies. Therefore, nonliving cell-derived techniques such as extracellular vesicle (EV) therapy and cell membrane-based therapy to fulfil the unmet demand for tissue repair are important. Nonliving cell-derived biomaterials are safer and more controllable, and their efficacy is easier to enhance through bioengineering approaches. Here, we described the development and evolution from cell therapy to EV therapy and cell membrane-based therapy for tissue repair. Furthermore, the latest advances in nonliving cell-derived therapies empowered by advanced engineering techniques are emphatically reviewed, and their potential and challenges in the future are discussed.
Keywords: Biomaterials; Cell membrane; Cell therapy; Extracellular vesicles; Regenerative medicine; Stem cells.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Xia H, Li X, Gao W, Fu X, Fang RH, Zhang L, et al. Tissue repair and regeneration with endogenous stem cells. Nat Rev Mater. 2018;3(7):174–193.
-
- Daar AS, Greenwood HL. A proposed definition of regenerative medicine. J Tissue Eng Regen Med. 2007;1(3):179–184. - PubMed
-
- Jenq RR, Van den Brink MR. Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nat Rev Cancer. 2010;10(3):213–221. - PubMed
-
- Chien KR, Frisén J, Fritsche-Danielson R, Melton DA, Murry CE, Weissman IL. Regenerating the field of cardiovascular cell therapy. Nat Biotechnol. 2019;37(3):232–237. - PubMed
-
- Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20(5):637–647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

