A protocol for the Development of Core Outcome Sets for Endodontic Treatment modalities (COSET): an international consensus process
- PMID: 34789318
- PMCID: PMC8597272
- DOI: 10.1186/s13063-021-05764-x
A protocol for the Development of Core Outcome Sets for Endodontic Treatment modalities (COSET): an international consensus process
Abstract
Background: The outcome of endodontic treatment is generally assessed using a range of patient and clinician-centred, non-standardised clinical and radiographic outcome measures. This makes it difficult to synthesise evidence for systematic analysis of the literature and the development of clinical guidelines. Core outcome sets (COS) represent a standardised list of outcomes that should be measured and reported in all clinical studies in a particular field. Recently, clinical researchers and guideline developers have focussed on the need for the integration of a patient-reported COS with clinician-centred measures. This study aims to develop a COS that includes both patient-reported outcomes and clinician-centred measures for various endodontic treatment modalities to be used in clinical research and practice.
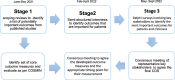
Methods: To identify reported outcomes (including when and how they are measured), systematic reviews and their included clinical studies, which focus on the outcome of endodontic treatment and were published between 1990 and 2020 will be screened. The COSs will be defined by a consensus process involving key stakeholders using semi-structured interviews and an online Delphi methodology followed by an interactive virtual consensus meeting. A heterogeneous group of key 'stakeholders' including patients, general dental practitioners, endodontists, endodontic teachers, clinical researchers, students and policy-makers will be invited to participate. Patients will establish, via interactive interviews, which outcomes they value and feel should be included in a COS. In the Delphi process, other stakeholders will be asked to prioritise outcomes identified from the literature and patient interviews and will have the opportunity at the end of the first round to add outcomes that are not included, but which they consider relevant. Feedback will be provided in the second round, when participants will be asked to prioritise the list again. If consensus is reached, the remaining outcomes will be discussed at an online meeting and agreement established via defined consensus rules of outcome inclusion. If consensus is not reached after the second round, a third round will be conducted with feedback, followed by the online meeting. Following the identification of a COS, we will proceed to identify how and when these outcomes are measured.
Discussion: Using a rigorous methodology, the proposed consensus process aims to develop a COS for endodontic treatment that will be relevant to stakeholders. The results of the study will be shared with participants and COS users. To increase COS uptake, it will also be actively shared with clinical guideline developers, research funders and the editors of general dental and endodontology journals.
Trial registration: COMET 1879. 21 May 2021.
Keywords: Clinical endpoints; Clinician-centred outcomes; Endodontics; Patient-reported outcomes; Root canal treatment; Surgical endodontics; Vital pulp treatment.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have competing interests.
References
MeSH terms
LinkOut - more resources
Full Text Sources