Clinical Practice Guideline: Recommendations on the In-hospital Treatment of Patients with COVID-19
- PMID: 34789365
- PMCID: PMC8948341
- DOI: 10.3238/arztebl.m2021.0374
Clinical Practice Guideline: Recommendations on the In-hospital Treatment of Patients with COVID-19
Abstract
Background: The mortality of COVID-19 patients who are admitted to a hospital because of the disease remains high. The implementation of evidence-based treatments can improve the quality of care.
Methods: The new clinical practice guideline is based on publications retrieved by a systematic search in the Medline databases via PubMed and in the Cochrane COVID-19 trial registry, followed by a structured consensus process leading to the adoption of graded recommendations.
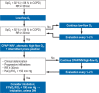
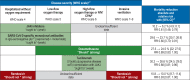
Results: Therapeutic anticoagulation can be considered in patients who do not require intensive care and have an elevated risk of thromboembolism (for example, those with D-dimer levels ≥ 2 mg/L). For patients in intensive care, therapeutic anticoagulation has no benefit. For patients with hypoxemic respiratory insufficiency, prone positioning and an early therapy attempt with CPAP/noninvasive ventilation (CPAP, continuous positive airway pressure) or high-flow oxygen therapy is recommended. Patients with IgG-seronegativity and, at most, low-flow oxygen should be treated with SARS-CoV-2-specific monoclonal antibodies (at present, casirivimab and imdevimab). Patients needing no more than low-flow oxygen should additionally be treated with janus kinase (JAK) inhibitors. All patients who need oxygen (low-flow, high-flow, noninvasive ventilation/CPAP, invasive ventilation) should be given systemic corticosteroids. Tocilizumab should be given to patients with a high oxygen requirement and progressively severe COVID-19 disease, but not in combination with JAK inhibitors.
Conclusion: Noninvasive ventilation, high-flow oxygen therapy, prone positioning, and invasive ventilation are important elements of the treatment of hypoxemic patients with COVID-19. A reduction of mortality has been demonstrated for the administration of monoclonal antibodies, JAK inhibitors, corticosteroids, tocilizumab, and therapeutic anticoagulation to specific groups of patients.
Figures
Comment in
-
In Reply.Dtsch Arztebl Int. 2022 Jun 24;119(25):439. doi: 10.3238/arztebl.m2022.0143. Dtsch Arztebl Int. 2022. PMID: 36178314 Free PMC article. No abstract available.
References
-
- Malin JJ, Spinner CD, Janssens U, et al. Key summary of German national treatment guidance for hospitalized COVID-19 patients: Key pharmacologic recommendations from a national German living guideline using an Evidence to Decision Framework (last updated 1705.2021) Infection. 2021:1–14. - PMC - PubMed
-
- AWMF. S3-Leitlinie: Empfehlungen zur stationären Therapie von Patienten mit COVID-19 - Living Guideline (Stand 10/2021). Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V. (AWMF) https://www.awmf.org/leitlinien/detail/ll/113-001LG.html (last accessed on 2 November 2021)
-
- Grieco DL, Menga LS, Cesarano M, et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: The HENIVOT randomized clinical trial. JAMA. 2021;325:1731–1743. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

