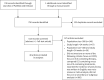
Discordant diagnostic criteria for pneumonia in COPD trials: a review
- PMID: 34789465
- PMCID: PMC9488621
- DOI: 10.1183/16000617.0124-2021
Discordant diagnostic criteria for pneumonia in COPD trials: a review
Abstract
Inhaled corticosteroids (ICS) have a class effect of increasing pneumonia risk in patients with COPD. However, pneumonia incidence varies widely across clinical trials of ICS use in COPD. This review clarifies methodological differences in defining and recording pneumonia events in these trials and discusses factors that could contribute to the varying pneumonia incidence. Literature searches and screening yielded 40 relevant references for inclusion. Methods used to capture pneumonia events in these studies included investigator-reported pneumonia adverse events, standardised list of signs or symptoms, radiographic confirmation of suspected cases and/or confirmation by an independent clinical end-point committee. In general, more stringent pneumonia diagnosis criteria led to lower reported pneumonia incidence rates. In addition, studies varied in design and population characteristics, including exacerbation history and lung function, factors that probably contribute to the varying pneumonia incidence. As such, cross-trial comparisons are problematic. A minimal set of standardised criteria for diagnosis and reporting of pneumonia should be used in COPD studies, as well as reporting of patients' pneumonia history at baseline, to allow comparison of pneumonia rates between trials. Currently, within-trial comparison of ICS-containing versus non-ICS-containing treatments is the appropriate method to assess the influence of ICS on pneumonia incidence.
Copyright ©The authors 2021.
Conflict of interest statement
Conflict of interest: R.A. Wise reports other and non-financial support from GlaxoSmithKline (funding the study and funding medical writing support by Anne Errichelli at Fishawack Indicia Ltd, UK), during the conduct of the study; grants and personal fees from AstraZeneca/Medimmune/Pearl (data monitoring committee, grants, consulting), grants and personal fees from Boehringer Ingelheim (steering committee, data monitoring committee, grants), personal fees from Contrafect (clinical end-point committee), grants and personal fees from AstraZeneca (research grant, consulting), grants and personal fees from GSK (research grant, consulting), personal fees from Sunovion (workshop, consulting), personal fees from Merck (data monitoring committee), personal fees from Verona (consultant), personal fees from Mylan/Theravance (consultant), personal fees from Propeller Health (consultant), grants and personal fees from GSK (scientific advisory board, clinical end-point committee, Data Monitoring Committee, research grant support), personal fees from Novartis (consultant), personal fees from ChimRix (data monitoring committee), personal fees from FSD Pharma (data monitoring committee), personal fees from AbbVie (data monitoring committee), personal fees from Bristol Myers Squibb (data monitoring committee), personal fees from Puretech (data monitoring committee), personal fees from Galderma (clinical end-point committee), personal fees from Chiesi (clinical end-point committee), outside the submitted work. Conflict of interest: M. Bafadhel reports other and non-financial support from GlaxoSmithKline (funding the study and funding medical writing support by Anne Errichelli at Fishawack Indicia Ltd, UK), during the conduct of the study; grants, personal fees and other from AstraZeneca (research grants, advisory board attendance, educational meeting attendance), personal fees and other from Chiesi (advisory board attendance, educational meeting attendance), personal fees and other from Boehringer Ingelheim (advisory board attendance, educational meeting attendance), personal fees and other from GlaxoSmithKline (advisory board attendance), personal fees and other from ProAxsis (scientific advisor), personal fees and other from AlbusHealth (scientific advisor), outside the submitted work. Conflict of interest: C. Crim reports other and non-financial support from GlaxoSmithKline (funding the study and funding medical writing support by Anne Errichelli at Fishawack Indicia Ltd, UK), during the conduct of the study; C. Crim is a former employee of GlaxoSmithKline and has shares/options held in GSK, outside the submitted work. Conflict of interest: G.J. Criner reports other and non-financial support from GlaxoSmithKline (funding the study and funding medical writing support by Anne Errichelli at Fishawack Indicia Ltd, UK), during the conduct of the study; personal fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, CSA Medical, Eolo, GlaxoSmithKline, HGE Technologies, Novartis, Nuvaira, Olympus, Pulmonx and Verona, outside the submitted work. Conflict of interest: N.C. Day reports other and non-financial support from GlaxoSmithKline (funding the study and funding medical writing support by Anne Errichelli at Fishawack Indicia Ltd, UK), during the conduct of the study; N.C. Day is an employee of GlaxoSmithKline and has shares/options held in GSK, outside the submitted work. Conflict of interest: D.M.G. Halpin reports other and non-financial support from GlaxoSmithKline (funding the study and funding medical writing support by Anne Errichelli at Fishawack Indicia Ltd, UK), during the conduct of the study; personal fees from AstraZeneca, personal fees and non-financial support from Boehringer Ingelheim, personal fees from Chiesi, personal fees from GlaxoSmithKline, personal fees and non-financial support from Novartis, personal fees from Pfizer, outside the submitted work. Conflict of interest: M.K. Han reports other and non-financial support from GlaxoSmithKline (funding the study and funding medical writing support by Anne Errichelli at Fishawack Indicia Ltd, UK), during the conduct of the study; outside the submitted work, M.K. Han also reports personal fees from GlaxoSmithKline, AstraZeneca, Boehringer Inhgelheim, Cipla, Chiesi, Novartis, Pulmonx, Teva, Verona, Merck, Mylan, Sanofi, DevPro, Aerogen, Polarian, Regeneron, United Therapeutics, Medscape and Integrity. She has received either in kind research support or funds paid to the institution from the NIH, Novartis, Sunovion, Nuvaira, Sanofi, AstraZeneca, Boehringer Ingelheim, Gala Therapeutics, Biodesix, the COPD Foundation and the American Lung Association. She has participated in Data Safety Monitoring Boards for Novartis and Medtronic with funds paid to the institution. Conflict of interest: P. Lange reports other and non-financial support from GlaxoSmithKline (funding the study and funding medical writing support by Anne Errichelli at Fishawack Indicia Ltd, UK), during the conduct of the study; personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, personal fees from GlaxoSmithKline, outside the submitted work. Conflict of interest: D.A. Lipson reports other and non-financial support from GlaxoSmithKline (funding the study and funding medical writing support by Anne Errichelli at Fishawack Indicia Ltd, UK), during the conduct of the study; D.A. Lipson is an employee of GlaxoSmithKline and has shares/options held in GSK, outside the submitted work. Conflict of interest: F.J. Martinez reports other and non-financial support from GlaxoSmithKline (funding the study and funding medical writing support by Anne Errichelli at Fishawack Indicia Ltd, UK), during the conduct of the study; other from Afferent/Merck (IPF Steering Committee (academic productivity)), personal fees, non-financial support and other from AstraZeneca (COPD Advisory Boards (personal fees honoraria and non-personal travel support), study steering committee (non-personal travel support), DSMB (other – academic co-authorship)), other from Bayer (ILD Steering Committee (academic productivity)), personal fees, non-financial support and other from Boehringer Ingelheim (COPD Advisory Board (personal fees and non-personal travel support); ATS presentation (personal fees); progressive pulmonary fibrosis DSMB (no financial support but travel); ERS IPF study result presentation (personal fee and travel support; IPF study steering committee chair), non-financial support and other from Bioscale/ProterrixBio (COPD scientific advisory board (no direct financial compensation, support for NIH study)), other from Bridge Biotherapeutics (IPF consultation), personal fees and non-financial support from Canadian Respiratory Network (COPD CME presentation (honorarium and travel support)), personal fees and non-financial support from Chiesi (COPD CME presentation (personal fees honoraria and non-personal travel support); Advisory Board (personal fees honoraria and travel support)), personal fees from France Foundation (IPF CME presentation (honorarium)), personal fees and non-financial support from Genentech (COPD advisory board (personal fee and non-personal travel support) and asthma DSMB (no support); IPF advisory board (personal fees honorarium and non-personal travel support)), other from Gilead (IPF study steering committee (other – co-authorship)), personal fees and non-financial support from GlaxoSmithKline (COPD advisory boards (personal fees honoaria and non-personal travel support), study steering committee (non-personal travel support), DSMB (other – academic co-authorship)), personal fees and non-financial support from Inova Fairfax Health System (COPD CME presentation (personal support honorarium and non-personal travel support)), personal fees from MD Magazine (COPD CME programme (personal fee honorarium and non-personal travel support)), personal fees and non-financial support from Methodist Hospital Brooklyn (IPF and COPD CME programs (personal fee honoraria)), personal fees and non-financial support from Miller Communications (COPD and IPF CME programs (personal fees honoraria and non-personal travel support)), non-financial support from Nitto (IPF Study Teleconference and Steering Committee (non-personal travel support)), personal fees and non-financial support from Novartis (COPD advisory board and international meeting COPD disease presentations (personal fees honoraria and non-personal travel support)), personal fees from New York University (ILD CME programme (personal fee honoraria)), personal fees and other from Patara (venture capital expert advice for IPF study (personal fees honoraria); IPF steering committee), personal fees and non-financial support from Pearl Pharmaceuticals (COPD advisory boards (personal fee honoraria and non-personal travel support) and COPD steering committee (academic productivity)), personal fees and non-financial support from PeerView Communications (COPD and IPF CME programmes (personal fees honoraria and non-personal travel support)), personal fees, non-financial support and other from Physicians Education Resource (IPF advisory board (personal fee honorarium and travel support)), personal fees from Prime Communications (COPD CME programme (personal fee honorarium)), other from Roche (IPF Steering Committee (academic productivity)), other from ProMetic (IPF steering committee (academic productivity)), personal fees from Rare Disease Health Communications (IPF CME programme (personal fee honorarium)), personal fees from Rockpointe (COPD CME programme (personal fee honorarium)), other from Biogen (IPF study DSMB (no support) and IPF study steering committee (academic productivity)), personal fees and non-financial support from Sunovion (COPD advisory boards (personal fee honoraria and non-personal travel support)), personal fees and non-financial support from Teva (COPD advisory board (personal fee honorarium and travel support)), personal fees and non-financial support from University of Alabama Birmingham (IPF CME presentation (personal fee honoraria and non-personal travel support)), personal fees from UpToDate (COPD CME (personal fee honoraria)), other from Veracyte (IPF study steering committee (other co-authorship)), personal fees from WebMD/MedScape (COPD and IPF CME presentations (personal fee honoraria)), non-financial support from Zambon (IPF study meeting (non-personal travel support) and advisory board (personal fees honorarium)), personal fees and non-financial support from American College of Chest Physicians, personal fees and non-financial support from ConCert, personal fees and non-financial support from Continuing Education, personal fees and non-financial support from National Society for Continuing Education, personal fees and non-financial support from Potomac, personal fees and non-financial support from Puerto Rico Respiratory Society, personal fees and non-financial support from Theravance, non-financial support from Nitto, personal fees from Columbia University, personal fees from Integras, personal fees from Unity, personal fees from Western Connecticut Health Network, personal fees from Academic CME, personal fees from Platform IQ, personal fees from American Thoracic Society, grants from Promedior, outside the submitted work. Conflict of interest: D.J. Maselli reports other and non-financial support from GlaxoSmithKline (funding the study and funding medical writing support by Anne Errichelli at Fishawack Indicia Ltd, UK), during the conduct of the study; personal fees from AstraZeneca, personal fees from Sanofi/Regeneron, personal fees from Amgen, outside the submitted work. Conflict of interest: D. Midwinter reports other and non-financial support from GlaxoSmithKline (funding the study and funding medical writing support by Anne Errichelli at Fishawack Indicia Ltd, UK), during the conduct of the study; D. Midwinter is an employee of GlaxoSmithKline and has shares/options held in GSK, outside the submitted work. Conflict of interest: D. Singh reports other and non-financial support from GlaxoSmithKline (funding the study and funding medical writing support by Anne Errichelli at Fishawack Indicia Ltd, UK), during the conduct of the study; personal fees from GlaxoSmithKline, grants and personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Chiesi, personal fees from Cipla, personal fees from Genentech, grants and personal fees from Glenmark, grants and personal fees from Menarini, grants and personal fees from Mundipharma, grants and personal fees from Novartis, personal fees from Peptinnovate, grants and personal fees from Pfizer, grants and personal fees from Pulmatrix, grants and personal fees from Theravance, grants and personal fees from Verona, outside the submitted work. Conflict of interest: M. Zysman reports other and non-financial support from GlaxoSmithKline (funding the study and funding medical writing support by Anne Errichelli at Fishawack Indicia Ltd, UK), during the conduct of the study; grants and personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, personal fees from Novartis, personal fees from Chiesi, personal fees from GlaxoSmithKline, personal fees from CSL Behring, outside the submitted work. Conflict of interest: M.T. Dransfield reports other and non-financial support from GlaxoSmithKline (funding the study and funding medical writing support by Anne Errichelli at Fishawack Indicia Ltd, UK), during the conduct of the study; other from Boehringer Ingelheim (consulting and contracted clinical trials), personal fees and other from GlaxoSmithKline (consulting and contracted clinical trials), personal fees and other from AstraZeneca (consulting and contracted clinical trials), other from PneumRx/BTG (contracted clinical trials), other from Pulmonx (contracted clinical trials), personal fees from Teva (consulting), other from Gala (contracted clinical trials), other from Nuvaira (contracted clinical trials), outside the submitted work. Conflict of interest: R.E.K. Russell reports other and non-financial support from GlaxoSmithKline (funding the study and funding medical writing support by Anne Errichelli at Fishawack Indicia Ltd, UK), during the conduct of the study; grants and personal fees from AstraZeneca, personal fees from GlaxoSmithKline, personal fees from Cipla, personal fees from Chiesi, personal fees from Boehringer Ingelheim, personal fees from Albus Health (Advisor), non-financial support from National Institute for Health Research Oxford Biomedical Research Centre, outside the submitted work.
Figures
References
-
- Metlay JP, Waterer GW, Long AC, et al. . Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200: e45–e67. doi:10.1164/rccm.201908-1581ST - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials