Real-World Effectiveness and Immunogenicity of BNT162b2 and mRNA-1273 SARS-CoV-2 Vaccines in Patients on Hemodialysis
- PMID: 34789546
- PMCID: PMC8763185
- DOI: 10.1681/ASN.2021060778
Real-World Effectiveness and Immunogenicity of BNT162b2 and mRNA-1273 SARS-CoV-2 Vaccines in Patients on Hemodialysis
Abstract
Background: Patients on hemodialysis have an elevated risk for COVID-19 but were not included in efficacy trials of SARS-CoV-2 vaccines.
Methods: We conducted a retrospective, observational study to estimate the real-world effectiveness and immunogenicity of two mRNA SARS-CoV-2 vaccines in a large, representative population of adult hemodialysis patients in the United States. In separate, parallel analyses, patients who began a vaccination series with BNT162b2 or mRNA-1273 in January and February 2021 were matched with unvaccinated patients and risk for outcomes were compared for days 1-21, 22-42, and ≥43 after first dose. In a subset of consented patients, blood samples were collected approximately 28 days after the second dose and anti-SARS-CoV-2 immunoglobulin G was measured.
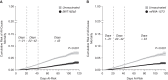
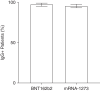
Results: A total of 12,169 patients received the BNT162b2 vaccine (matched with 44,377 unvaccinated controls); 23,037 patients received the mRNA-1273 vaccine (matched with 63,243 unvaccinated controls). Compared with controls, vaccinated patients' risk of being diagnosed with COVID-19 postvaccination became progressively lower during the study period (hazard ratio and 95% confidence interval for BNT162b2 was 0.21 [0.13, 0.35] and for mRNA-1273 was 0.27 [0.17, 0.42] for days ≥43). After a COVID-19 diagnosis, vaccinated patients were significantly less likely than unvaccinated patients to be hospitalized (for BNT162b2, 28.0% versus 43.4%; for mRNA-1273, 37.2% versus 45.6%) and significantly less likely to die (for BNT162b2, 4.0% versus 12.1%; for mRNA-1273, 5.6% versus 14.5%). Antibodies were detected in 98.1% (309/315) and 96.0% (308/321) of BNT162b2 and mRNA-1273 patients, respectively.
Conclusions: In patients on hemodialysis, vaccination with BNT162b2 or mRNA-1273 was associated with a lower risk of COVID-19 diagnosis and lower risk of hospitalization or death among those diagnosed with COVID-19. SARS-CoV-2 antibodies were detected in nearly all patients after vaccination. These findings support the use of these vaccines in this population.
Keywords: BNT162 vaccine; COVID-19; ESRD; RNA; SARS-CoV-2; coronavirus disease 2019; dialysis; end stage kidney disease; end-stage renal disease; messenger; vaccine.
Copyright © 2022 by the American Society of Nephrology.
Figures



References
-
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC: Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 324: 782–793, 2020 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

