Empirical identification and validation of tumor-targeting T cell receptors from circulation using autologous pancreatic tumor organoids
- PMID: 34789550
- PMCID: PMC8601084
- DOI: 10.1136/jitc-2021-003213
Empirical identification and validation of tumor-targeting T cell receptors from circulation using autologous pancreatic tumor organoids
Erratum in
-
Correction: Empirical identification and validation of tumor-targeting T cell receptors from circulation using autologous pancreatic tumor organoids.J Immunother Cancer. 2023 May;11(5):e003213corr1. doi: 10.1136/jitc-2021-003213corr1. J Immunother Cancer. 2023. PMID: 37188396 Free PMC article. No abstract available.
Abstract
Background: Tumor-specific cytotoxic T cells and T cell receptors are effective tools for cancer immunotherapy. Most efforts to identify them rely on known antigens or lymphocytes that have infiltrated into the tumor bed. Approaches to empirically identify tumor-targeting T cells and T cell receptors by exploiting all antigens expressed on tumor cell surfaces are not well developed for most carcinomas, including pancreatic cancer.
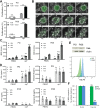
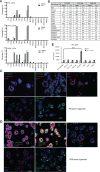
Methods: Autologous tumor organoids were stimulated with T cells from the patients' peripheral blood for 2 weeks to generate the organoid-primed T (opT) cells. opT cell phenotype was analyzed by monitoring changes in the expression levels of 28 cell surface and checkpoint proteins. Expression of ligands of the immune checkpoints was investigated by immunohistochemistry staining. T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and assayed by flow cytometry to monitor tumor-induced T cell proliferation changes. opT cell-mediated killing of three-dimensional organoids was measured using an M30 ELISA kit. T cell receptors (TCRs) were identified by deep sequencing of gDNA isolated from T cells, and the TCR specificity was confirmed by transferring TCRs to the T cell line SKW-3 or donor T cells.
Results: The co-culture was effective in the generation of CD8 + or CD4+opT cells. The opT cells killed autologous tumors in a granzyme B or Fas-Fas ligand-dependent manner and expressed markers of tissue-resident memory phenotype. Each patient-derived opT cell culture displayed a unique complement of checkpoint proteins. Interestingly, only NKG2A blockade showed a potent increase in the interferon-γ production compared with blocking programmed cell death protein 1 (PD-1) or programmed cell death ligand 1 (PD-L1) or TIM3 or TIGIT or LAG3. Importantly, TCR sequencing demonstrated a dramatic clonal expansion of T cells with a restricted subset of TCRs. Cloning and transferring the TCRs to heterologous T cells was sufficient to confer tumor cell recognition and cytotoxic properties in a patient-specific manner.
Conclusion: We report a platform for expanding tumor-targeting T cells from the peripheral blood of patients with pancreatic cancer. We identify the NKG2A-HLA-E axis as a potentially important checkpoint for CD8 +T cells for pancreatic cancer. Lastly, we demonstrate empirical identification of tumor-targeting TCRs that can be used for TCR-therapeutics.
Keywords: antigen-mediated; clonal selection; gastrointestinal neoplasms; immunologic techniques.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: LH and SKM have a patent application pending for the PTOM media used in this study (US20170267977A1). SKM consults for KAHR Medical. MH has stock and ownership interests in Champions Oncology, PharmaCyte Biotech, Bioncotech, Nelum, and Agenus; honoraria include Takeda, Agenus, InxMed, Pharmacyte, BioOncotech, Tolero, Novartis, Oncomatrix, KAHR Medical; he has a consulting or advisory role for Takeda, Agenus, InxMed, Pharmacyte, BioOncotech, Tolero, Novartis, Oncomatrix, KAHR Medical; his patents, royalties, and other intellectual property include: Myriad Genetics. Other authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
