Detection of cancer stem cells by EMT-specific biomarker-based peptide ligands
- PMID: 34789743
- PMCID: PMC8599855
- DOI: 10.1038/s41598-021-01138-0
Detection of cancer stem cells by EMT-specific biomarker-based peptide ligands
Abstract
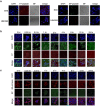
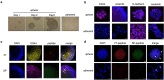
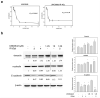
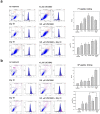
The occurrence of epithelial-mesenchymal transition (EMT) within tumors, which enables invasion and metastasis, is linked to cancer stem cells (CSCs) with drug and radiation resistance. We used two specific peptides, F7 and SP peptides, to detect EMT derived cells or CSCs. Human tongue squamous carcinoma cell line-SAS transfected with reporter genes was generated and followed by spheroid culture. A small molecule inhibitor-Unc0642 and low-dose ionizing radiation (IR) were used for induction of EMT. Confocal microscopic imaging and fluorescence-activated cell sorting analysis were performed to evaluate the binding ability and specificity of peptides. A SAS xenograft mouse model with EMT induction was established for assessing the binding affinity of peptides. The results showed that F7 and SP peptides not only specifically penetrated into cytoplasm of SAS cells but also bound to EMT derived cells and CSCs with high nucleolin and vimentin expression. In addition, the expression of CSC marker and the binding of peptides were increased in tumors isolated from Unc0642/IR-treated groups. Our study demonstrates the potential of these peptides for detecting EMT derived cells or CSCs and might provide an alternative isolation method for these subpopulations within the tumor in the future.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
EGFR inhibitors prevent induction of cancer stem-like cells in esophageal squamous cell carcinoma by suppressing epithelial-mesenchymal transition.Cancer Biol Ther. 2015;16(6):933-40. doi: 10.1080/15384047.2015.1040959. Epub 2015 Apr 21. Cancer Biol Ther. 2015. PMID: 25897987 Free PMC article.
-
Cancer stem-like cells enriched with CD29 and CD44 markers exhibit molecular characteristics with epithelial-mesenchymal transition in squamous cell carcinoma.Arch Dermatol Res. 2013 Jan;305(1):35-47. doi: 10.1007/s00403-012-1260-2. Epub 2012 Jun 28. Arch Dermatol Res. 2013. PMID: 22740085
-
Inhibitory effects of metformin at low concentration on epithelial-mesenchymal transition of CD44(+)CD117(+) ovarian cancer stem cells.Stem Cell Res Ther. 2015 Dec 30;6:262. doi: 10.1186/s13287-015-0249-0. Stem Cell Res Ther. 2015. PMID: 26718286 Free PMC article.
-
Clinical Evolution of Epithelial-Mesenchymal Transition in Human Carcinomas.Cancer Res. 2020 Jan 15;80(2):304-318. doi: 10.1158/0008-5472.CAN-18-3539. Epub 2019 Nov 15. Cancer Res. 2020. PMID: 31732654 Free PMC article.
-
Tumor-targeted fluorescence labeling systems for cancer diagnosis and treatment.Cancer Sci. 2022 Jun;113(6):1919-1929. doi: 10.1111/cas.15369. Epub 2022 Apr 18. Cancer Sci. 2022. PMID: 35398956 Free PMC article. Review.
Cited by
-
The silent spread: exploring diverse metastatic pathways in high-grade serous ovarian cancer.Front Med (Lausanne). 2025 Mar 5;12:1539024. doi: 10.3389/fmed.2025.1539024. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40109727 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

