Population receptive fields of human primary visual cortex organised as DC-balanced bandpass filters
- PMID: 34789812
- PMCID: PMC8599479
- DOI: 10.1038/s41598-021-01891-2
Population receptive fields of human primary visual cortex organised as DC-balanced bandpass filters
Abstract
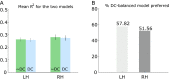
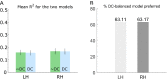
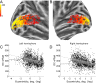
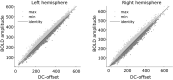
The response to visual stimulation of population receptive fields (pRF) in the human visual cortex has been modelled with a Difference of Gaussians model, yet many aspects of their organisation remain poorly understood. Here, we examined the mathematical basis and signal-processing properties of this model and argue that the DC-balanced Difference of Gaussians (DoG) holds a number of advantages over a DC-biased DoG. Through functional magnetic resonance imaging (fMRI) pRF mapping, we compared performance of DC-balanced and DC-biased models in human primary visual cortex and found that when model complexity is taken into account, the DC-balanced model is preferred. Finally, we present evidence indicating that the BOLD signal DC offset contains information related to the processing of visual stimuli. Taken together, the results indicate that V1 pRFs are at least frequently organised in the exact constellation that allows them to function as bandpass filters, which makes the separation of stimulus contrast and luminance possible. We further speculate that if the DoG models stimulus contrast, the DC offset may reflect stimulus luminance. These findings suggest that it may be possible to separate contrast and luminance processing in fMRI experiments and this could lead to new insights on the haemodynamic response.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Modeling center-surround configurations in population receptive fields using fMRI.J Vis. 2012 Mar 8;12(3):10. doi: 10.1167/12.3.10. J Vis. 2012. PMID: 22408041
-
A second-order orientation-contrast stimulus for population-receptive-field-based retinotopic mapping.Neuroimage. 2018 Jan 1;164:183-193. doi: 10.1016/j.neuroimage.2017.06.073. Epub 2017 Jun 27. Neuroimage. 2018. PMID: 28666882
-
A new method for estimating population receptive field topography in visual cortex.Neuroimage. 2013 Nov 1;81:144-157. doi: 10.1016/j.neuroimage.2013.05.026. Epub 2013 May 16. Neuroimage. 2013. PMID: 23684878
-
Abnormal visual field maps in human cortex: a mini-review and a case report.Cortex. 2014 Jul;56:14-25. doi: 10.1016/j.cortex.2012.12.005. Epub 2012 Dec 21. Cortex. 2014. PMID: 23347557 Review.
-
Human cortical areas underlying the perception of optic flow: brain imaging studies.Int Rev Neurobiol. 2000;44:269-92. doi: 10.1016/s0074-7742(08)60746-1. Int Rev Neurobiol. 2000. PMID: 10605650 Review.
Cited by
-
Two-Dimensional Population Receptive Field Mapping of Human Primary Somatosensory Cortex.Brain Topogr. 2023 Nov;36(6):816-834. doi: 10.1007/s10548-023-01000-8. Epub 2023 Aug 27. Brain Topogr. 2023. PMID: 37634160 Free PMC article.
-
Orientation selectivity properties for the affine Gaussian derivative and the affine Gabor models for visual receptive fields.J Comput Neurosci. 2025 Mar;53(1):61-98. doi: 10.1007/s10827-024-00888-w. Epub 2025 Jan 29. J Comput Neurosci. 2025. PMID: 39878929 Free PMC article.
-
Do the receptive fields in the primary visual cortex span a variability over the degree of elongation of the receptive fields?J Comput Neurosci. 2025 Jun 20. doi: 10.1007/s10827-025-00907-4. Online ahead of print. J Comput Neurosci. 2025. PMID: 40540232
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

