Tandem Mass Tag labelling quantitative acetylome analysis of differentially modified proteins during mycoparasitism of Clonostachys chloroleuca 67-1
- PMID: 34789861
- PMCID: PMC8599485
- DOI: 10.1038/s41598-021-01956-2
Tandem Mass Tag labelling quantitative acetylome analysis of differentially modified proteins during mycoparasitism of Clonostachys chloroleuca 67-1
Abstract
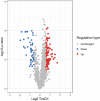
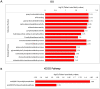
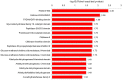
Lysine acetylation (Kac) is an important post-translational modification (PTM) of proteins in all organisms, but its functions have not been extensively explored in filamentous fungi. In this study, a Tandem Mass Tag (TMT) labelling lysine acetylome was constructed, and differentially modified Kac proteins were quantified during mycoparasitism and vegetative growth in the biocontrol fungus Clonostachys chloroleuca 67-1, using liquid chromatography-tandem mass spectrometry (LC-MS/MS). A total of 1448 Kac sites were detected on 740 Kac proteins, among which 126 sites on 103 proteins were differentially regulated. Systematic bioinformatics analyses indicate that the modified Kac proteins were from multiple subcellular localizations and involved in diverse functions including chromatin assembly, glycometabolism and redox activities. All Kac sites were characterized by 10 motifs, including the novel CxxKac motif. The results suggest that Kac proteins may have effects of broadly regulating protein interaction networks during C. chloroleuca parasitism to Sclerotinia sclerotiorum sclerotia. This is the first report of a correlation between Kac events and the biocontrol activity of C. chloroleuca. Our findings provide insight into the molecular mechanisms underlying C. chloroleuca control of plant fungal pathogens regulated by Kac proteins.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Global Insight into Lysine Acetylation Events and Their Links to Biological Aspects in Beauveria bassiana, a Fungal Insect Pathogen.Sci Rep. 2017 Mar 15;7:44360. doi: 10.1038/srep44360. Sci Rep. 2017. PMID: 28295016 Free PMC article.
-
Global Proteome Analysis Links Lysine Acetylation to Diverse Functions in Oryza Sativa.Proteomics. 2018 Jan;18(1). doi: 10.1002/pmic.201700036. Proteomics. 2018. PMID: 29106068
-
The Mitogen-Activated Protein Kinase Gene Crmapk Is Involved in Clonostachys chloroleuca Mycoparasitism.Mol Plant Microbe Interact. 2020 Jul;33(7):902-910. doi: 10.1094/MPMI-03-20-0062-R. Epub 2020 Jun 2. Mol Plant Microbe Interact. 2020. PMID: 32282260
-
The transcription factor-encoding gene crtf is involved in Clonostachys chloroleuca mycoparasitism on Sclerotinia sclerotiorum.Microbiol Res. 2018 May;210:6-11. doi: 10.1016/j.micres.2018.03.002. Epub 2018 Mar 8. Microbiol Res. 2018. PMID: 29625660
-
Protein lysine acetylation analysis: current MS-based proteomic technologies.Analyst. 2013 Mar 21;138(6):1628-36. doi: 10.1039/c3an36837h. Epub 2013 Jan 30. Analyst. 2013. PMID: 23361263 Review.
Cited by
-
Quantitative Acetylome Analysis of Differentially Modified Proteins in Virulence-Differentiated Fusarium oxysporum f. sp. cucumerinum Isolates during Cucumber Colonization.J Fungi (Basel). 2023 Sep 13;9(9):920. doi: 10.3390/jof9090920. J Fungi (Basel). 2023. PMID: 37755028 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- 2019YFD1002000/National Key Research and Development Program of China
- 2019YFD1002000/National Key Research and Development Program of China
- 2019YFD1002000/National Key Research and Development Program of China
- 2019YFD1002000/National Key Research and Development Program of China
- CARS-23-D05/China Agriculture Research System
- CARS-23-D05/China Agriculture Research System
- CARS-23-D05/China Agriculture Research System
- CARS-23-D05/China Agriculture Research System
- CARS-23-D05/China Agriculture Research System
- zdzx2018009/Science and Technology Major Project of Inner Mongolia
- zdzx2018009/Science and Technology Major Project of Inner Mongolia
- zdzx2018009/Science and Technology Major Project of Inner Mongolia
- 2019-NK-116/Science and Technology Program of Qinghai
- 2019-NK-116/Science and Technology Program of Qinghai
LinkOut - more resources
Full Text Sources

