Ainsliadimer C, a disesquiterpenoid isolated from Ainsliaea macrocephala, ameliorates inflammatory responses in adipose tissue via Sirtuin 1-NLRP3 inflammasome axis
- PMID: 34789920
- PMCID: PMC9253034
- DOI: 10.1038/s41401-021-00797-z
Ainsliadimer C, a disesquiterpenoid isolated from Ainsliaea macrocephala, ameliorates inflammatory responses in adipose tissue via Sirtuin 1-NLRP3 inflammasome axis
Abstract
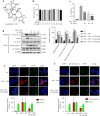
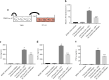
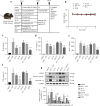
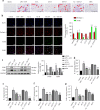
Interleukin-1β (IL-1β), a key pro-inflammatory cytokine, is majorly produced by macrophages through NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome, which has been identified as the culprit to deteriorate the inflammatory crosstalk between macrophages and adipocytes. Ainsliadimer C (AC) is a disesquiterpenoid isolated from Ainsliaea macrocephala. In the current study, we investigated the effects of AC on adipose tissue inflammation in co-culture of macrophages and adipocytes in vitro as well as in LPS-treated mice in vivo. We showed that AC (20-80 µM) dose-dependently inhibited the secretion of IL-1β from LPS plus ATP-stimulated THP-1 macrophages by inhibiting the activation of NLRP3 inflammasome. Furthermore, we found that AC treatment activated NAD+-dependent deacetylase Sirtuin 1 (SIRT1), resulting in reduced acetylation level of NLRP3. Molecular modeling analysis revealed that binding of AC to sirtuin-activating compound-binding domain increased the affinity of the substrate to the catalytic domain of SIRT1. Moreover, AC (80 µM) significantly attenuated macrophage-conditioned medium-induced inflammatory responses in 3T3-L1 adipocytes. In LPS-induced acute inflammatory mice, administration of AC (20, 60 mg·kg-1·d-1, ip) for 5 days significantly suppressed the pro-inflammatory cytokine levels in serum and epididymal white adipose tissue (eWAT), attenuated macrophage infiltration into eWAT, and mitigated adipose tissue inflammation. The beneficial effects of AC were blocked by co-administration of a selective SIRT1 inhibitor EX-527 (10 mg·kg-1·d-1). Taken together, AC suppresses NLRP3-mediated IL-1β secretion through activating SIRT1, leading to attenuated inflammation in macrophages and adipose tissue, which might be a candidate to treat obesity-associated metabolic diseases.
Keywords: Ainsliadimer C; EX-527; NLRP3 inflammasome; Sirtuin 1; adipose tissue inflammation; dexamethasone; interleukin-1β; macrophages; metabolic disorders; obesity.
© 2021. The Author(s), under exclusive licence to CPS and SIMM.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

