Respiration-Driven Brain Oscillations in Emotional Cognition
- PMID: 34790100
- PMCID: PMC8592085
- DOI: 10.3389/fncir.2021.761812
Respiration-Driven Brain Oscillations in Emotional Cognition
Abstract
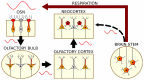
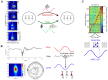
Respiration paces brain oscillations and the firing of individual neurons, revealing a profound impact of rhythmic breathing on brain activity. Intriguingly, respiration-driven entrainment of neural activity occurs in a variety of cortical areas, including those involved in higher cognitive functions such as associative neocortical regions and the hippocampus. Here we review recent findings of respiration-entrained brain activity with a particular focus on emotional cognition. We summarize studies from different brain areas involved in emotional behavior such as fear, despair, and motivation, and compile findings of respiration-driven activities across species. Furthermore, we discuss the proposed cellular and network mechanisms by which cortical circuits are entrained by respiration. The emerging synthesis from a large body of literature suggests that the impact of respiration on brain function is widespread across the brain and highly relevant for distinct cognitive functions. These intricate links between respiration and cognitive processes call for mechanistic studies of the role of rhythmic breathing as a timing signal for brain activity.
Keywords: embodied cognition; emotion; network; neuronal circuits; neuronal synchronization; oscillations; respiration; slow oscillation.
Copyright © 2021 Folschweiller and Sauer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

