Risk of Adverse Events After Anti-TNF Treatment for Inflammatory Rheumatological Disease. A Meta-Analysis
- PMID: 34790122
- PMCID: PMC8591221
- DOI: 10.3389/fphar.2021.746396
Risk of Adverse Events After Anti-TNF Treatment for Inflammatory Rheumatological Disease. A Meta-Analysis
Abstract
Background: Adalimumab, golimumab, infliximab, certolizumab, and etanercept are five anti-tumor necrosis factor (anti-TNF) medicines that have been approved for use in rheumatology. Apart from their well-established therapeutic usefulness, -it is unclear to what extent -they are linked to an increased risk of various side effects. The present meta-analysis was carried out to assess the risk of infection and other side effects after anti-TNF- α for the treatment of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Methods: We searched PubMed, Cinahl (via Ebsco), Scopus, and Web of Sciences databases for trials comparing anti-TNF medications to placebo or no therapy in adult patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis from August 2006 to August 2020. A total of 23 articles were used for meta-analysis. The Cochrane Collaboration's risk of bias tool was used to assess the methodological quality of the included studies. In addition, a random-effects model was used to calculate the pooled odds ratio, and Forest plots were constructed to determine the risk of infections and cancer following the use of anti-TNF treatment. Results: Treatment with anti-TNFα agents resulted in an increase in the risk of serious infections (OR: 1.72, 95% CI: 1.56-1.90, p < 0.00001) and an increase in cancer risk (OR: 1.36, 95% CI: 1.20-1.53, p < 0.00001) whereas the risk of developing tuberculosis was not significantly different with anti-TNFα agents versus those without treatment with anti-TNFα agents (OR: 2.55, 95% CI: 0.40-16.23, p = 0.32) although the number of studies is limited to make a definitive conclusion. The risk of bias of the included studies was unclear to high across most domains, and there was evidence of publication bias for most outcomes. Conclusion: The present meta-analysis suggests an increased risk of infectious adverse events, including overall adverse events and cancer following anti-TNFα treatment, whereas the risk of tuberculosis was not significantly different. Although anti-TNF agents have shown promise to treat inflammatory conditions, their use should be balanced by the risk-benefit ratio as suggested by the meta-analysis.
Keywords: ankylosing spondylitis; anti TNF therapy; malignancy; psoriatic arthritis (artritis psoriatica); rheumatoid arthritis; risk of infections.
Copyright © 2021 Li, Zhang, Wu, Zhou, Meng and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





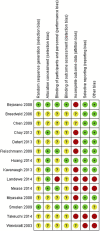
References
-
- Abbott Laboratories (2012) Humira (Adalimumab) [highlights of Prescribing Information]. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/ (accessed December 15, 2020).
-
- Axelrad J., Bernheim O., Colombel J. F., Malerba S., Ananthakrishnan A., Yajnik V., et al. (2016). Risk of New or Recurrent Cancer in Patients with Inflammatory Bowel Disease and Previous Cancer Exposed to Immunosuppressive and Anti-tumor Necrosis Factor Agents. Clin. Gastroenterol. Hepatol. 14 (1), 58–64. 10.1016/j.cgh.2015.07.037 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

