Remote Delivery of Motor Function Assessment and Training for Clinical Trials in Neuromuscular Disease: A Response to the COVID-19 Global Pandemic
- PMID: 34790223
- PMCID: PMC8592083
- DOI: 10.3389/fgene.2021.735538
Remote Delivery of Motor Function Assessment and Training for Clinical Trials in Neuromuscular Disease: A Response to the COVID-19 Global Pandemic
Abstract
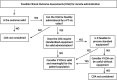
Clinical outcome assessments of function or strength, assessed by physical therapists, are commonly used as primary endpoints in clinical trials, natural history studies and within clinics for individuals with neuromuscular disorders. These evaluations not only inform the efficacy of investigational agents in clinical trials, but also importantly track disease trajectory to prospectively advise need for equipment, home and work modifications, and other assistive devices. The COVID-19 pandemic had a global impact on the safety and feasibility of in-person visits and assessments, necessitating rapid development of mitigation strategies to ensure ongoing collection of key clinical trial endpoints and access to expert clinical care despite travel restrictions. Physical therapists who are expert in neuromuscular disorders working across clinics, countries, and clinical trials developed initial guidelines and methods for the suitability and feasibility of performing remote evaluations. A number of Sponsors introduced amendments to their study protocols to enable remote evaluations, supported by live video streaming of the assessment to their local clinical evaluators. Similarly, application of these techniques to clinical telemedicine enabled objective evaluations for use in payer discussions, equipment procurement, and general access to expert physical therapy services. Here we report on our methodology for adapting current practices to remote testing and considerations for remote evaluations.
Keywords: COVID-19; clinical outcome assessment (COA); clinical trials; natural history; neuromuscular disorders (NMD); physical therapy; telemedicine.
Copyright © 2021 James, Rose, Alfano, Reash, Eagle and Lowes.
Conflict of interest statement
MJ provides consultancy services for the following companies: ATOM International (covers consultancy services provided to Amicus Therapeutics Pty Ltd., Ascendis Pharma, Biomarin, Catabasis, Faraday, FibroGen, Genethon, Italfarmaco, NS Pharma, Pfizer, PTC Therapeutics, QED Therapeutics Ltd., Reveragen, Sarepta Therapeutics). MJ has received payment for participation on Advisory Boards for F. Hoffman La Roche AG, PTC Therapeutics and fee support for PhD studies from the Jain Foundation. KR provides consultancy services for the following companies: ATOM International (covers consultancy services provided to Amicus Therapeutics Pty Ltd., Ascendis Pharma, BioMarin, Catabasis, FibroGen, Italfarmaco, NS Pharma, Pfizer, PTC Therapeutics, QED Therapeutics, Ltd., Sarepta Therapeutics and Summit Pharmaceuticals International) as well as consultancy services provided independently to Biogen and F. Hoffman La Roche AG. KR also receives payment for participation on Advisory Boards and steering committees and for assisting to deliver education initiatives for Biogen and F. Hoffman La Roche AG. LA reports royalties and other support from Sarepta Therapeutics, royalties for licensed technologies, other support from Novartis Gene Therapies, personal fees from Biogen. Also provides consultancy services for ATOM International (Amicus Therapeutics Pty Ltd., Catabasis, Genethon, Italfarmaco, NS Pharma, Pfizer, PTC Therapeutics), outside the submitted work. NR reports support from Sarepta Therapeutics and as a trainer for ATOM International, Ltd. (Amicus Therapeutics Pty Ltd., Catabasis, Genethon, Italfarmaco, NS Pharma, Pfizer, PTC Therapeutics), outside the submitted work. LL reports royalties and other support from Sarepta Therapeutics, royalties for licensed technologies, other support from Novartis Gene Therapies, personal fees from Biogen and as a trainer for ATOM International (Amicus Therapeutics Pty Ltd., Catabasis, Genethon, Italfarmaco, NS Pharma, Pfizer, PTC Therapeutics), outside the submitted work. ME is the Managing Director of ATOM International (covers consultancy services provided to Amicus Therapeutics Pty Ltd., Ascendis Pharma, Biomarin, Capricor Catabasis, Denali, Faraday, FibroGen, Genethon, Italfarmaco, Lysogene, Modis, NS Pharma, Pfizer, PTC Therapeutics, QED Therapeutics, Ltd., Reveragen, Sarepta Therapeutics, Solid Biosciences).
Figures
References
-
- Alfano L. N., Miller N. F., Berry K. M., Yin H., Rolf K. E., Flanigan K. M., et al. (2017). The 100-meter Timed Test: Normative Data in Healthy Males and Comparative Pilot Outcome Data for Use in Duchenne Muscular Dystrophy Clinical Trials. Neuromuscul. Disord. 27, 452–457. 10.1016/j.nmd.2017.02.007 - DOI - PubMed
-
- EMA, Europen Medicines Agency (2020). Guidance on the Management of Clinical Trials During the Covid-19 (Coronavirus) Pandemic. Available at: https://ec.europa.eu/health/sites/default/files/files/eudralex/vol-10/gu... (Accessed March 9th, 2021).
-
- FDA, U.S. Department of Health and Human Services (2020). Food and Drug Administration U.S. 2020. 'Conduct of Clinical Trials of Medical Products During the COVID-19 Public Health Emergency Guidance for Industry, Investigators, and Institutional Review Boards. March 2020 Updated on January 27, 2021'. Available at: https://www.fda.gov/media/136238/download (Accessed March 9th, 2021).
LinkOut - more resources
Full Text Sources