Efficacy of bowel preparation regimens for colon capsule endoscopy: a systematic review and meta-analysis
- PMID: 34790528
- PMCID: PMC8589531
- DOI: 10.1055/a-1529-5814
Efficacy of bowel preparation regimens for colon capsule endoscopy: a systematic review and meta-analysis
Abstract
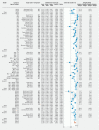
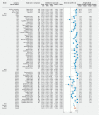
Background and study aims Colon capsule endoscopy (CCE) is an alternative to conventional colonoscopy (CC) in specific clinical settings. High completion rates (CRs) and adequate cleanliness rates (ACRs) are fundamental quality parameters if CCE is to be widely implemented as a CC equivalent diagnostic modality. We conducted a systematic review and meta-analysis to investigate the efficacy of different bowel preparations regimens on CR and ACR in CCE. Patients and methods We performed a systematic literature search in PubMed, Embase, CINAHL, Web of Science, and the Cochrane Library. Data were independently extracted per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The primary outcome measures (CR, ACR) were retrieved from the individual studies and pooled event rates were calculated. Results Thirty-four observational (OBS) studies (n = 3,789) and 12 randomized clinical trials (RCTs) (n = 1,214) comprising a total 5,003 patients were included. The overall CR was 0.798 (95 % CI, 0.764-0.828); the highest CRs were observed with sodium phosphate (NaP) + gastrografin booster (n = 2, CR = 0.931, 95 % CI, 0.820-0.976). The overall ACR was 0.768 (95 % CI, 0.735-0.797); the highest ACRs were observed with polyethylene glycol (PEG) + magnesium citrate (n = 4, ER = 0.953, 95 % CI, 0.896-0.979). Conclusions In the largest meta-analysis on CCE bowel preparation regimens, we found that both CRs and ACRs are suboptimal compared to the minimum recommended standards for CC. PEG laxative and NaP booster were the most commonly used but were not associated with higher CRs or ACRs. Well-designed studies on CCE should be performed to find the optimal preparation regimen.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
Competing interests Dr. Koulaouzidis is consultant for Jinshan. He is director of iCERV Ltd and cofounder of AJM Medicaps Ltd. He has received a Given Imaging Ltd-ESGE grant, and material support for clinical research from SynMed/Intromedic. In the last 10 years, he has received honoraria and lecture fees from Jinshan, Dr Falk Pharma UK, and Ferring. He has also received educational travel support from Aquilant, Jinshan, Dr Falk Pharma, Almirall, Ferring, and has participated in advisory board meetings for Tillots, Ankon, and Dr Falk PharmaUK. Dr. Toth has received research grant from the Swedish Cancer Society and the Swedish ALF-agreement.
Figures







References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

