Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy
- PMID: 34790633
- PMCID: PMC8591095
- DOI: 10.3389/fped.2021.723532
Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy
Abstract
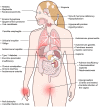
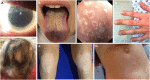
Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED), also known as autoimmune polyglandular syndrome type-1 (APS-1), is a rare monogenic autoimmune disease caused by loss-of-function mutations in the autoimmune regulator (AIRE) gene. AIRE deficiency impairs immune tolerance in the thymus and results in the peripheral escape of self-reactive T lymphocytes and the generation of several cytokine- and tissue antigen-targeted autoantibodies. APECED features a classic triad of characteristic clinical manifestations consisting of chronic mucocutaneous candidiasis (CMC), hypoparathyroidism, and primary adrenal insufficiency (Addison's disease). In addition, APECED patients develop several non-endocrine autoimmune manifestations with variable frequencies, whose recognition by pediatricians should facilitate an earlier diagnosis and allow for the prompt implementation of targeted screening, preventive, and therapeutic strategies. This review summarizes our current understanding of the genetic, immunological, clinical, diagnostic, and treatment features of APECED.
Keywords: AIRE; APECED syndrome; APS-1; IFN-γ; IL-17; autoantibodies; self-reactive T cells; type-I interferons.
Copyright © 2021 Ferré, Schmitt and Lionakis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

