The RNA Methyltransferase METTL3 Promotes Endothelial Progenitor Cell Angiogenesis in Mandibular Distraction Osteogenesis via the PI3K/AKT Pathway
- PMID: 34790657
- PMCID: PMC8591310
- DOI: 10.3389/fcell.2021.720925
The RNA Methyltransferase METTL3 Promotes Endothelial Progenitor Cell Angiogenesis in Mandibular Distraction Osteogenesis via the PI3K/AKT Pathway
Abstract
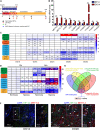
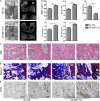
Distraction osteogenesis (DO) is used to treat large bone defects in the field of oral and maxillofacial surgery. Successful DO-mediated bone regeneration is dependent upon angiogenesis, and endothelial progenitor cells (EPCs) are key mediators of angiogenic processes. The N6-methyladenosine (m6A) methyltransferase has been identified as an important regulator of diverse biological processes, but its role in EPC-mediated angiogenesis during DO remains to be clarified. In the present study, we found that the level of m6A modification was significantly elevated during the process of DO and that it was also increased in the context of EPC angiogenesis under hypoxic conditions, which was characterized by increased METTL3 levels. After knocking down METTL3 in EPCs, m6A RNA methylation, proliferation, tube formation, migration, and chicken embryo chorioallantoic membrane (CAM) angiogenic activity were inhibited, whereas the opposite was observed upon the overexpression of METTL3. Mechanistically, METTL3 silencing reduced the levels of VEGF and PI3Kp110 as well as the phosphorylation of AKT, whereas METTL3 overexpression reduced these levels. SC79-mediated AKT phosphorylation was also able to restore the angiogenic capabilities of METTL3-deficient EPCs in vitro and ex vivo. In vivo, METTL3-overexpressing EPCs were additionally transplanted into the DO callus, significantly enhancing bone regeneration as evidenced by improved radiological and histological manifestations in a canine mandibular DO model after consolidation over a 4-week period. Overall, these results indicate that METTL3 accelerates bone regeneration during DO by enhancing EPC angiogenesis via the PI3K/AKT pathway.
Keywords: METTL3; PI3K/AKT signaling pathway; angiogenesis; distraction osteogenesis; endothelial progenitor cells.
Copyright © 2021 Jiang, Zhu, Huang, Zhao, Zhang, An, Liao, Guo, Liu, Zhou and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Cetrulo C. L., Jr., Knox K. R., Brown D. J., Ashinoff R. L., Dobryansky M., Ceradini D. J., et al. (2005). Stem cells and distraction osteogenesis: endothelial progenitor cells home to the ischemic generate in activation and consolidation. Plast. Reconstr. Surg. 116 1053–1064; discussion 1065–1057. 10.1097/01.prs.0000178403.79051.70 - DOI - PubMed
LinkOut - more resources
Full Text Sources

