Effects of SGLT2 Inhibitors on Renal Outcomes in Patients With Chronic Kidney Disease: A Meta-Analysis
- PMID: 34790672
- PMCID: PMC8591237
- DOI: 10.3389/fmed.2021.728089
Effects of SGLT2 Inhibitors on Renal Outcomes in Patients With Chronic Kidney Disease: A Meta-Analysis
Abstract
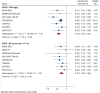
Introduction: The effects of sodium-glucose cotransporter-2 (SGLT2) inhibitors on renal outcomes in patients with chronic kidney disease (CKD) were initially demonstrated in recent trials. However, the magnitude of renal benefits for CKD patients with different baseline features and underlying diseases remains unclear. Method: We systematically searched the Embase, PubMed, Web of Science, and Cochrane library databases from inception to April 15, 2021 to identify eligible trials. The primary outcome was a composite of worsening kidney function, end-stage kidney disease (ESKD), or renal death. Efficacy and safety outcomes were stratified by baseline features, such as type 2 diabetes, heart failure, atherosclerotic cardiovascular disease, proteinuria, and renal function. Results: A total of nine studies were included. These studies included 25,749 patients with estimated glomerular filtration rate (eGFR)<60 mL/min/1.73 m2 and 12,863 patients with urine albumin-to-creatinine ratio (UACR) >300 mg/g. SGLT2 inhibitors reduced the risk of the primary renal outcome by 30% in patients with eGFR<60 mL/min/1.73 m2 (HR 0.70, [95% CI 0.58-0.83], I2 = 0.00%) and by 43% in patients with UACR > 300 mg/g (HR 0.57, [95% CI 0.48-0.67], I2 = 16.59%). A similar benefit was observed in CKD patients with type 2 diabetes. SGLT2 inhibitors had no clear effects on renal outcomes in patients with eGFR<60 mL/min/1.73 m2 combined with atherosclerotic cardiovascular disease (HR 0.74, [95% CI 0.51-1.06], I2 = 0.00%). However, they reduced the risk of major renal outcomes by 46% (HR 0.54, [95% CI 0.38-0.76], I2 = 0.00%) in patients with atherosclerotic cardiovascular disease and macroalbuminuria (defined as UACR > 300 mg/g). SGLT2 inhibitors did not significantly reduce the risk of major renal outcomes in CKD patients with heart failure (eGFR<60 mL/min/1.73 m2: HR 0.81, [95% CI 0.47-1.38], I2 = 0.00%; UACR > 300 mg/g: HR 0.66, [95% CI 0.41-1.07], I2 = 0.00%). SGLT2 inhibitors showed consistent renal benefits across different levels of eGFR (P interaction = 0.48). Conclusion: SGLT2 inhibitors significantly reduced the risk of the primary outcome in CKD patients. However, for patients with different features and underlying diseases, there exists differences in the renal protective effect.
Keywords: SGLT2 inhibitors; chronic kidney disease; meta-analysis; protective effect; renal outcome.
Copyright © 2021 Li, Lv, Zhu, Wei, Gui, Liu, Zhou, Zheng, Zhou and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
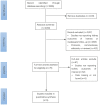
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

