A novel prognostic signature for idiopathic pulmonary fibrosis based on five-immune-related genes
- PMID: 34790776
- PMCID: PMC8576669
- DOI: 10.21037/atm-21-4545
A novel prognostic signature for idiopathic pulmonary fibrosis based on five-immune-related genes
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) is a highly fatal lung disease of unknown etiology with a median survival after diagnosis of only 2-3 years. Its poor prognosis is due to the limited therapy options available as well as the lack of effective prognostic indicators. This study aimed to construct a novel prognostic signature for IPF to assist in the personalized management of IPF patients during treatment.
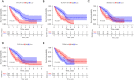
Methods: Differentially-expressed genes (DEGs) in IPF patients versus healthy individuals were analyzed using the "limma" package of R software. Immune-related genes (IRGs) were obtained from the ImmPort database. Univariate Cox regression analysis was adopted to screen significantly prognostic IRGs for IPF patients. Multiple Cox regression analysis was used to identify optimal prognostic IRGs and construct a prognostic signature.
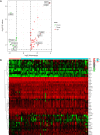
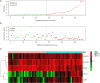
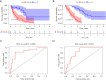
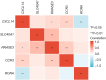
Results: Compared with healthy individuals, there were a total of 52 prognosis-related DEGs in the bronchoalveolar lavage (BAL) samples of IPF patients, of which 37 genes were identified as IRGs. Of these, five genes (CXCL14, SLC40A1, RNASE3, CCR3, and RORA) were significantly associated with overall survival (OS) in IPF patients, and were utilized for establishment of the prognostic signature. IPF patients were divided into high- and low-risk groups based on the prognostic signature. Marked differences in the OS probability were observed between high- and low-risk IPF patients. The area under curves (AUCs) of the receiver operating characteristic (ROC) curve for the prognostic signature in the training and validation cohorts were 0.858 and 0.837, respectively. The expression levels between RNASE3 and SLC40A1 (P<0.01, r=0.394), between RORA and CXCL14 (P<0.01, r=-0.355), between CCR3 and CXCL14 (P<0.01, r=0.258), as well as between RNASE3 and CCR3 (P<0.01, r=0.293) were significantly correlated.
Conclusions: We developed a validated and reproducible IRG-based prognostic signature that should be helpful in the personalized management of patients with IPF, providing new insights into the relationship between the immune system and IPF.
Keywords: GEO; Idiopathic pulmonary fibrosis (IPF); bronchoalveolar lavage (BAL); immune-related genes (IRGs); prognostic signature.
2021 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-4545). The authors have no conflicts of interest to declare.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
