Optimal biomechanical parameters for measuring sclerotic chronic graft-versus-host disease
- PMID: 34790906
- PMCID: PMC8594905
- DOI: 10.1016/j.xjidi.2021.100037
Optimal biomechanical parameters for measuring sclerotic chronic graft-versus-host disease
Abstract
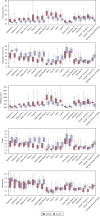
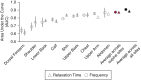
Skin biomechanical parameters (dynamic stiffness, frequency, relaxation time, creep, and decrement) measured using a myotonometer (MyotonPRO) could inform management of sclerotic disease. To determine which biomechanical parameter(s) can accurately differentiate sclerotic chronic graft-versus-host disease (cGVHD) patients from post-hematopoietic cell transplant (post-HCT) controls, 15 sclerotic cGVHD patients and 11 post-HCT controls were measured with the myotonometer on 18 anatomic sites. Logistic regression and two machine learning algorithms, LASSO regression and random forest, were developed to classify subjects. In univariable analysis, frequency had the highest overfit-corrected area under the receiver operating characteristic curve (AUC 0.91). Backward stepwise selection and random forest machine learning identified frequency and relaxation time as the optimal parameters for differentiating sclerotic cGVHD patients from post-HCT controls. LASSO regression selected the combination of frequency and relaxation time (overfit-corrected AUC 0.87). Discriminatory ability was maintained when only the sites accessible while the patient is supine (12 sites) were used. We report the distribution of values for these highly discriminative biomechanical parameters, which could inform assessment of disease severity in future quantitative biomechanical studies of sclerotic cGVHD.
Conflict of interest statement
Conflict of interest: A.V. is the inventor of the Myoton but has no financial interest in the device.
Figures

References
-
- Arai S., Arora M., Wang T., Spellman S.R., He W., Couriel D.R., et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21:266–274. - PMC - PubMed
-
- Breiman L., Cutler A., Liaw A., Wiener M. randomForest: Breiman and Cutler’s random forests for classification and regression. 2018. https://cran.r-project.org/web/packages/randomForest/index.html (accessed 6 July, 2020)
-
- Carpenter P.A., Kitko C.L., Elad S., Flowers M.E., Gea-Banacloche J.C., Halter J.P., et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: V. The 2014 ancillary therapy and supportive care working group report. Biol Blood Marrow Transplant. 2015;21:1167–1187. - PMC - PubMed
-
- Chen F., Dellalana L.E., Gandelman J.S., Vain A., Jagasia M.H., Tkaczyk E.R. Non-invasive measurement of sclerosis in cutaneous cGVHD patients with the handheld device Myoton: a cross-sectional study [published correction appears in Bone Marrow Transplant 2020;55:992] Bone Marrow Transplant. 2019;54:616–619. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

