The neuroradiology of progressive multifocal leukoencephalopathy: a clinical trial perspective
- PMID: 34791056
- PMCID: PMC9630710
- DOI: 10.1093/brain/awab419
The neuroradiology of progressive multifocal leukoencephalopathy: a clinical trial perspective
Abstract
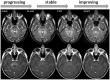
Progressive multifocal leukoencephalopathy (PML) is an opportunistic infection of the CNS caused by the JC virus, which infects white and grey matter cells and leads to irreversible demyelination and neuroaxonal damage. Brain MRI, in addition to the clinical presentation and demonstration of JC virus DNA either in the CSF or by histopathology, is an important tool in the detection of PML. In clinical practice, standard MRI pulse sequences are utilized for screening, diagnosis and monitoring of PML, but validated imaging-based outcome measures for use in prospective, interventional clinical trials for PML have yet to be established. We review the existing literature regarding the use of MRI and PET in PML and discuss the implications of PML histopathology for neuroradiology. MRI not only demonstrates the localization and extent of PML lesions, but also mirrors the tissue destruction, ongoing viral spread, and resulting inflammation. Finally, we explore the potential for imaging measures to serve as an outcome in PML clinical trials and provide recommendations for current and future imaging outcome measure development in this area.
Keywords: clinical trial design; imaging outcome measures; magnetic resonance imaging; progressive multifocal leukoencephalopathy.
Published by Oxford University Press on behalf of the Guarantors of Brain 2021. This work is written by a US Government employee and is in the public domain in the US.
Figures



References
-
- Brew BJ, Davies NW, Cinque P, Clifford DB, Nath A. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol. 2010;6(12):667–679. - PubMed
-
- Bowen LN, Smith B, Reich D, Quezado M, Nath A. HIV-associated opportunistic CNS infections: Pathophysiology, diagnosis and treatment. Nat Rev Neurol. 2016;12(11):662–674. - PubMed
-
- Major EO, Yousry TA, Clifford DB. Pathogenesis of progressive multifocal leukoencephalopathy and risks associated with treatments for multiple sclerosis: A decade of lessons learned. Lancet Neurol. 2018;17(5):467–480. - PubMed
-
- Wijburg MT, Warnke C, McGuigan C, et al. Pharmacovigilance during treatment of multiple sclerosis: Early recognition of CNS complications. J Neurol Neurosurg Psychiatry. 2021;92(2):177–188. - PubMed

