Long-term outcome of targeted therapy of underlying conditions in patients with early persistent atrial fibrillation and heart failure: data of the RACE 3 trial
- PMID: 34791160
- PMCID: PMC9282914
- DOI: 10.1093/europace/euab270
Long-term outcome of targeted therapy of underlying conditions in patients with early persistent atrial fibrillation and heart failure: data of the RACE 3 trial
Abstract
Aims: The Routine vs. Aggressive risk factor driven upstream rhythm Control for prevention of Early persistent atrial fibrillation (AF) in heart failure (HF) (RACE 3) trial demonstrated that targeted therapy of underlying conditions improved sinus rhythm maintenance at 1 year. We now explored the effects of targeted therapy on the additional co-primary endpoints; sinus rhythm maintenance and cardiovascular outcome at 5 years.
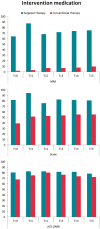
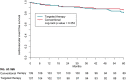
Methods and results: Patients with early persistent AF and mild-to-moderate stable HF were randomized to targeted or conventional therapy. Both groups received rhythm control therapy according to guidelines. The targeted group additionally received four therapies: angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers (ARBs), statins, mineralocorticoid receptor antagonists (MRAs), and cardiac rehabilitation. The presence of sinus rhythm and cardiovascular morbidity and mortality at 5-year follow-up were assessed. Two hundred and sixteen patients consented for long-term follow-up, 107 were randomized to targeted and 109 to conventional therapy. At 5 years, MRAs [76 (74%) vs. 10 (9%) patients, P < 0.001] and statins [81 (79%) vs. 59 (55%), P < 0.001] were used more in the targeted than conventional group. Angiotensin-converting enzyme inhibitors/ARBs and physical activity were not different between groups. Sinus rhythm was present in 49 (46%) targeted vs. 43 (39%) conventional group patients at 5 years (odds ratio 1.297, lower limit of 95% confidence interval 0.756, P = 0.346). Cardiovascular mortality and morbidity occurred in 20 (19%) in the targeted and 15 (14%) conventional group patients, P = 0.353.
Conclusion: In patients with early persistent AF and HF superiority of targeted therapy in sinus rhythm maintenance could not be preserved at 5-year follow-up. Cardiovascular outcome was not different between groups.
Trial registration number: Clinicaltrials.gov NCT00877643.
Keywords: Atrial fibrillation; Cardiovascular outcome; Early persistent atrial fibrillation; Heart failure; Rhythm control; Risk factors.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures


References
-
- Dudink EAMP, Erküner Ö, Berg J, Nieuwlaat R, de Vos CB, Weijs Bet al. . The influence of progression of atrial fibrillation on quality of life: a report from the Euro Heart Survey. Europace 2018;20:929–34. - PubMed
-
- Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens Let al. ; CASTLE-AF Investigators . Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. - PubMed
-
- Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan Aet al. ; EAST-AFNET 4 Trial Investigators . Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

