Azithromycin in patients with COVID-19: a systematic review and meta-analysis
- PMID: 34791330
- PMCID: PMC8690025
- DOI: 10.1093/jac/dkab404
Azithromycin in patients with COVID-19: a systematic review and meta-analysis
Abstract
Background: Azithromycin has been widely used in the management of COVID-19. However, the evidence on its actual effects remains disperse and difficult to apply in clinical settings. This systematic review and meta-analysis summarizes the available evidence to date on the beneficial and adverse effects of azithromycin in patients with COVID-19.
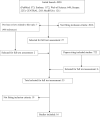
Methods: The PRISMA 2020 statement criteria were followed. Randomized controlled trials (RCTs) and observational studies comparing clinical outcomes of patients treated with and without azithromycin, indexed until 5 July 2021, were searched in PubMed, Embase, The Web of Science, Scopus, The Cochrane Central Register of Controlled Trials and MedRXivs. We used random-effects models to estimate pooled effect size from aggregate data.
Results: The initial search produced 4950 results. Finally, 16 studies, 5 RCTs and 11 with an observational design, with a total of 22 984 patients, were included. The meta-analysis showed no difference in mortality for those treated with or without azithromycin, in observational studies [OR: 0.90 (0.66-1.24)], RCTs [OR: 0.97 (0.87-1.08)] and also when both types of studies were pooled together [with an overall OR: 0.95 (0.79-1.13)]. Different individual studies also reported no significant difference for those treated with or without azithromycin in need for hospital admission or time to admission from ambulatory settings, clinical severity, need for intensive care, or adverse effects.
Conclusions: The results presented in this systematic review do not support the use of azithromycin in the management of COVID-19. Future research on treatment for patients with COVID-19 may need to focus on other drugs.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


Similar articles
-
Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis.Clin Microbiol Infect. 2021 Jan;27(1):19-27. doi: 10.1016/j.cmi.2020.08.022. Epub 2020 Aug 26. Clin Microbiol Infect. 2021. PMID: 32860962 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Vitamin D supplementation for the treatment of COVID-19: a living systematic review.Cochrane Database Syst Rev. 2021 May 24;5(5):CD015043. doi: 10.1002/14651858.CD015043. Cochrane Database Syst Rev. 2021. PMID: 34029377 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review.Cochrane Database Syst Rev. 2020 May 14;5(5):CD013600. doi: 10.1002/14651858.CD013600. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Jul 10;7:CD013600. doi: 10.1002/14651858.CD013600.pub2. PMID: 32406927 Free PMC article. Updated.
-
Proactive Prophylaxis With Azithromycin and HydroxyChloroquine in Hospitalised Patients With COVID-19 (ProPAC-COVID): A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jun 10;21(1):513. doi: 10.1186/s13063-020-04409-9. Trials. 2020. PMID: 32522282 Free PMC article.
Cited by
-
Ultra-Low-Dose Inhalation of Melphalan as an Additional Treatment for COVID-19-Associated Pneumonia.J Clin Med. 2025 Mar 21;14(7):2149. doi: 10.3390/jcm14072149. J Clin Med. 2025. PMID: 40217600 Free PMC article.
-
Antibiotic Treatment in Patients Hospitalized for Nonsevere COVID-19.JAMA Netw Open. 2025 May 1;8(5):e2511499. doi: 10.1001/jamanetworkopen.2025.11499. JAMA Netw Open. 2025. PMID: 40388163 Free PMC article.
-
Effectiveness of a clinical decision support algorithm (CDSA) on reducing unnecessary antibiotic prescriptions for upper respiratory tract infections among ambulatory HIV-infected adults in Mozambique: a cluster randomized controlled trial.Res Sq [Preprint]. 2025 Jul 2:rs.3.rs-6972996. doi: 10.21203/rs.3.rs-6972996/v1. Res Sq. 2025. PMID: 40630534 Free PMC article. Preprint.
-
A Comprehensive Review on the Efficacy of Several Pharmacologic Agents for the Treatment of COVID-19.Life (Basel). 2022 Nov 1;12(11):1758. doi: 10.3390/life12111758. Life (Basel). 2022. PMID: 36362912 Free PMC article. Review.
-
Natural and Synthetic Coumarins as Potential Drug Candidates against SARS-CoV-2/COVID-19.Curr Med Chem. 2025;32(3):539-562. doi: 10.2174/0109298673285609231220111556. Curr Med Chem. 2025. PMID: 38243979 Review.
References
-
- Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ. et al. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther 2014; 143: 225–45. - PubMed
-
- International Prospective Register of Systematic Reviews. (PROSPERO) https://www.crd.york.ac.uk/PROSPERO/.

