Immunogenicity of Fractional Dose Inactivated Poliovirus Vaccine in India
- PMID: 34791350
- PMCID: PMC8865014
- DOI: 10.1093/jpids/piab091
Immunogenicity of Fractional Dose Inactivated Poliovirus Vaccine in India
Abstract
Introduction: Following the withdrawal of Sabin type 2 from trivalent oral poliovirus vaccine (tOPV) in 2016, the introduction of ≥1 dose of inactivated poliovirus vaccine (IPV) in routine immunization was recommended, either as 1 full dose (0.5mL, intramuscular) or 2 fractional doses of IPV (fIPV-0.1mL, intradermal). India opted for fIPV. We conducted a comparative assessment of IPV and fIPV.
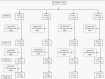
Methods: This was a 4-arm, open-label, multicenter, randomized controlled trial. Infants were enrolled and vaccines administered according to the study design, and the blood was drawn at age 6, 14, and 18 weeks for neutralization testing against all 3 poliovirus types.
Results: Study enrolled 799 infants. The seroconversion against type 2 poliovirus with 2 fIPV doses was 85.8% (95% confidence interval [CI]: 80.1%-90.0%) when administered at age 6 and 14 weeks, 77.0% (95% CI: 70.5-82.5) when given at age 10 and 14 weeks, compared to 67.9% (95% CI: 60.4-74.6) following 1 full-dose IPV at age 14 weeks.
Conclusion: The study demonstrated the superiority of 2 fIPV doses over 1 full-dose IPV in India. Doses of fIPV given at 6 and 14 weeks were more immunogenic than those given at 10 and 14 weeks. Clinical Trial Registry of India (CTRI). Clinical trial registration number was CTRI/2017/02/007793.
Keywords: EPI schedule; India; fractional dose inactivated poliovirus vaccine; immunogenicity.
© The Author(s) 2021. Published by Oxford University Press on behalf of The Journal of the Pediatric Infectious Diseases Society.
Figures


References
-
- Forty-First World Health Assembly. WHA41.28 – global eradication of poliomyelitis by the year 2000 [Internet]. Geneva; 1988. Accessed February 4, 2020. polioresolution4128.pdf
-
- World Health Organization. WHO South-East Asia Region certified polio-free [Internet]. SEARO. Accessed September 11, 2019. http://www.searo.who.int/mediacentre/releases/2014/pr1569/en/
-
- WHO. Poliomyelitis [Internet]. Accessed December 20, 2019. https://www.who.int/news-room/fact-sheets/detail/poliomyelitis
-
- GPEI-Endemic Countries [Internet]. Accessed September 16, 2019. http://polioeradication.org/where-we-work/polio-endemic-countries/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

