Potentiation of plant defense by bacterial outer membrane vesicles is mediated by membrane nanodomains
- PMID: 34791473
- PMCID: PMC8846181
- DOI: 10.1093/plcell/koab276
Potentiation of plant defense by bacterial outer membrane vesicles is mediated by membrane nanodomains
Abstract
Outer membrane vesicles (OMVs) are released from the outer membranes of Gram-negative bacteria during infection and modulate host immunity during host-pathogen interactions. The mechanisms by which OMVs are perceived by plants and affect host immunity are unclear. Here, we used the pathogen Xanthomonas campestris pv. campestris to demonstrate that OMV-plant interactions at the Arabidopsis thaliana plasma membrane (PM) modulate various host processes, including endocytosis, innate immune responses, and suppression of pathogenesis by phytobacteria. The lipid phase of OMVs is highly ordered and OMVs directly insert into the Arabidopsis PM, thereby enhancing the plant PM's lipid order; this also resulted in strengthened plant defenses. Strikingly, the integration of OMVs into the plant PM is host nanodomain- and remorin-dependent. Using coarse-grained simulations of molecular dynamics, we demonstrated that OMV integration into the plant PM depends on the membrane lipid order. Our computational simulations further showed that the saturation level of the OMV lipids could fine-tune the enhancement of host lipid order. Our work unraveled the mechanisms underlying the ability of OMVs produced by a plant pathogen to insert into the host PM, alter host membrane properties, and modulate plant immune responses.
© American Society of Plant Biologists 2021. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

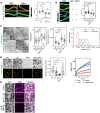
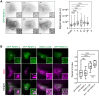



Comment in
-
Back to the roots: A focus on plant cell biology.Plant Cell. 2022 Jan 20;34(1):1-3. doi: 10.1093/plcell/koab278. Plant Cell. 2022. PMID: 34755878 Free PMC article. No abstract available.
References
-
- Abel NB, Buschle CA, Hernandez-Ryes C, Burkart SS, Deroubaix A-F, Mergner J, Gronnier J, Jarsch IK, Folgmann J, Braun KH (2021) A hetero-oligomeric remorin-receptor complex regulates plant development. bioRxiv, 10.1101/2021.01.28.428596 (January 29, 2021) - DOI
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1: 19–25
-
- Adani F, Papa G, Schievano A, Cardinale G, D’Imporzano G, Tambone F (2011) Nanoscale structure of the cell wall protecting cellulose from enzyme attack. Environ Sci Technol 45: 1107–1113 - PubMed
-
- Albers P, Üstün S, Witzel K, Kraner M, Börnke F (2019) A remorin from Nicotiana benthamiana interacts with the Pseudomonas type-III effector protein HopZ1a and is phosphorylated by the immune-related kinase PBS1. Mol Plant-Microbe Interact 32: 1229–1242 - PubMed
-
- Arnarez C, Uusitalo JJ, Masman MF, Ingólfsson HI, De Jong DH, Melo MN, Periole X, De Vries AH, Marrink SJ (2015) Dry Martini, a coarse-grained force field for lipid membrane simulations with implicit solvent. J Chem Theory Comput 11: 260–275 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

