Trends in the use of oral anticoagulants, antiplatelets and statins in four European countries: a population-based study
- PMID: 34791521
- PMCID: PMC8818635
- DOI: 10.1007/s00228-021-03250-6
Trends in the use of oral anticoagulants, antiplatelets and statins in four European countries: a population-based study
Abstract
Purpose: To evaluate time trends in the prevalence of antithrombotic and statin use in four European countries.
Methods: Using population-based data from the United Kingdom, Denmark, Spain and Italy between 2010 and 2018, we calculated standardized annual prevalence proportions of antithrombotics and statin use, and changes in prevalence proportions (2018 vs. 2010).
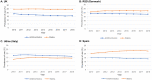
Results: Prevalence proportion of statins increased from 24.8% to 24.6% (UK), 21.0% to 22.3% (Region of Southern Denmark [RSD]), 12.9% to 14.3% (Udine, Italy), and 20.3% to 23.2% (Spain). Prevalence proportions of antithrombotics declined in all four countries: 18.7% to 15.9% (UK; - 2.8% points), 18.9% to 18.1% (RSD; - 0.8% points), 17.7% to 16.6% (Udine; - 1.1% points) and 15.0% to 13.6% (Spain; - 1.4% points). These declines were driven by reductions in low-dose aspirin use: 15.3% to 8.9% (UK; - 6.4% points), 16.3% to 9.5% (RSD; - 6.8% points), 13.5% to 11.6% (Udine; - 1.9% points), and 10.2% to 8.8% (Spain; - 1.4% points). In the UK, low-dose aspirin use declined from 9.1% to 4.3% (- 4.8% points) for primary CVD prevention, and from 49.6% to 36.9% (- 12.7% points) for secondary prevention. Oral anticoagulant use gradually increased but did not fully account for the decrease in low-dose aspirin use.
Conclusions: Antithrombotic use in the UK, RSD, Udine and Spain declined between 2010 and 2018, driven by a reduction in use of low-dose aspirin that is not completely explained by a gradual increase in OAC use. Use of statins remained constant in the UK, and increased gradually in the RSD, Udine and Spain.
Keywords: Anticoagulants; Antithrombotics; Aspirin; Database; Statins; Trends.
© 2021. The Author(s).
Conflict of interest statement
LAGR works for CEIFE, which has received research funding Bayer AG. LAGR has also received honoraria for serving on advisory boards for Bayer AG. DG has received honoraria from AstraZeneca (Sweden) for participation as a coinvestigator on a research project outside the submitted work; and receiving speaker honorarium from Bristol-Myers Squibb outside the submitted work. FdA has received unrestricted research grants from Instituto de Salud Carlos III, Instituto Teófilo Hernando, Chiesi and Sanofi-Pasteur, for other research projects. JH has participated in research projects funded by Alcon, Almirall, Astellas, Astra-Zeneca, Boehringer-Ingelheim, Novo Nordisk, Servier and LEO Pharma, all with funds paid to the institution where he was employed (no personal fees) and with no relation to the current study. LCS, SR-M, MG, CC and FV declare no conflicts of interest. PV and MS-G are employees of Bayer AG.
Figures



References
-
- Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31(19):2369–2429. doi: 10.1093/eurheartj/ehq278. - DOI - PubMed
-
- Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37(3):267–315. doi: 10.1093/eurheartj/ehv320ehv320[pii]. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

