N-Acylethanolamine acid amidase (NAAA) is dysregulated in colorectal cancer patients and its inhibition reduces experimental cancer growth
- PMID: 34791641
- PMCID: PMC9303321
- DOI: 10.1111/bph.15737
N-Acylethanolamine acid amidase (NAAA) is dysregulated in colorectal cancer patients and its inhibition reduces experimental cancer growth
Abstract
Background and purpose: N-Acylethanolamine acid amidase (NAAA) is a lysosomal enzyme accountable for the breakdown of N-acylethanolamines (NAEs) and its pharmacological inhibition has beneficial effects in inflammatory conditions. The knowledge of NAAA in cancer is fragmentary with an unclarified mechanism, whereas its contribution to colorectal cancer (CRC) is unknown to date.
Experimental approach: CRC xenograft and azoxymethane models were used to assess the in vivo effect of NAAA inhibition. Further, the tumour secretome was evaluated by an oncogenic array, CRC cell lines were used for in vitro studies, cell cycle was analysed by cytofluorimetry, NAAA was knocked down with siRNA, human biopsies were obtained from surgically resected CRC patients, gene expression was measured by RT-PCR and NAEs were measured by LC-MS.
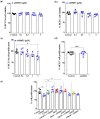
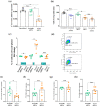
Key results: The NAAA inhibitor AM9053 reduced CRC xenograft tumour growth and counteracted tumour development in the azoxymethane model. NAAA inhibition affected the composition of the tumour secretome inhibiting the expression of EGF family members. In CRC cells, AM9053 reduced proliferation with a mechanism mediated by PPAR-α and TRPV1. AM9053 induced cell cycle arrest in the S phase associated with cyclin A2/CDK2 down-regulation. NAAA knock-down mirrored the effects of NAAA inhibition with AM9053. NAAA expression was down-regulated in human CRC tissues, with a consequential augmentation of NAE levels and dysregulation of some of their targets.
Conclusion and implications: Our results show novel data on the functional importance of NAAA in CRC progression and the mechanism involved. We propose that this enzyme is a valid drug target for the treatment of CRC growth and development.
Keywords: acylethanolamides; colon cancer; endocannabinoid system.
© 2021 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Abbracchio, M. P. , Alexander, W. , Al‐Hosaini, K. , Bäck, M. , Barnes, N. M. , Bathgate, R. , et al. (2021). The Concise Guide to PHARMACOLOGY 2021/22: G protein‐coupled receptors. British Journal of Pharmacology, 178(Suppl 1), S27–S156. - PubMed
-
- Alexander, S. P. , Cidlowski, J. A. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Coons, L. , Fuller, P. J. , Korach, K. S. , & Young, M. J. (2021). The Concise Guide to PHARMACOLOGY 2021/22: Nuclear hormone receptors. British Journal of Pharmacology, 178(Suppl 1), S246–S263. - PMC - PubMed
-
- Alexander, S. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Buneman, O. P. , Cidlowski, J. A. , Christopoulos, A. , Davenport, A. P. , Fabbro, D. , Spedding, M. , Striessnig, J. , Davies, J. A. , Ahlers‐Dannen, K. E. , … Zolghadri, Y. (2021). The Concise Guide to PHARMACOLOGY 2021/22: Introduction and other protein targets. British Journal of Pharmacology, 178(Suppl 1), S1–S26. - PMC - PubMed
-
- Alexander, S. P. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Aldrich, R. W. , Attali, B. , Baggetta, A. M. , Becirovic, E. , Biel, M. , Bill, R. M. , & Catterall, W. A. (2021). The Concise Guide to PHARMACOLOGY 2021/22: Ion channels. British Journal of Pharmacology, 178(Suppl 1), S157–S245. - PubMed
-
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , Cirino, G. , Docherty, J. R. , Giembycz, M. A. , Hoyer, D. , Insel, P. A. , Izzo, A. A. , Ji, Y. , MacEwan, D. J. , Mangum, J. , Wonnacott, S. , & Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

