Cutaneous and Allergic reactions due to COVID-19 vaccinations: A review
- PMID: 34791757
- PMCID: PMC8661794
- DOI: 10.1111/jocd.14613
Cutaneous and Allergic reactions due to COVID-19 vaccinations: A review
Abstract
Introduction: The pandemic caused by the novel coronavirus disease 2019 (COVID-19) has had an unprecedented impact on the overall health and the global economy. Vaccination is currently the most dependable strategy to end the pandemic, despite the slower-than-hoped-for rollout, particularly for low-to-middle-income countries, and the uncertain duration of protection afforded by vaccination. The spike protein of the virus (immunodominant antigen of the virus) is the main target of the approved and candidate SARS-CoV-2 vaccines. This protein binds to the ACE2 receptor of the host cell, initiating the entry of the virus into the cell and the chain of subsequent events ending to Acute Respiratory Distress Syndrome. The safety profile of these vaccines needs is closely assessed.
Methods: This comprehensive review includes searching the PubMed, EMBASE, and Web of Science databases using the keywords "coronavirus", "COVID-19", "vaccine", "cutaneous reactions", "allergic reactions", and "SARS-CoV-2". Manual searching of reference lists of included articles augmented the research. The research was updated in June 2021.
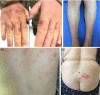
Results: In this narrative review, we tried to investigate and discuss the cutaneous and allergic reactions related to SARS-CoV-2 vaccines currently available in the literature. As a result, although COVID-19 vaccines can be reported to develop allergic and anaphylactic reactions, especially after m-RNA vaccines, they remain at a low rate, and it is observed that these reactions may develop more frequently, especially in patients with previous allergies and mast cell disorders. Fortunately, these reactions are generally transient, benign, self-limited.
Conclusion: Although there is still no definitive evidence, as dermatologists, we must be aware of the possibility of cutaneous reactions, newly diagnosed dermatoses, or exacerbation of existing dermatoses that may develop after the COVID-19 vaccinations.
Keywords: BNT162b2; COVID-19; SARS-CoV-2; allergic reactions; cutaneous reactions; vaccination.
© 2021 Wiley Periodicals LLC.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous