Smad3 Promotes Cancer-Associated Fibroblasts Generation via Macrophage-Myofibroblast Transition
- PMID: 34791825
- PMCID: PMC8728853
- DOI: 10.1002/advs.202101235
Smad3 Promotes Cancer-Associated Fibroblasts Generation via Macrophage-Myofibroblast Transition
Abstract
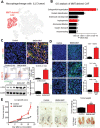
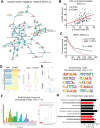
Cancer-associated fibroblasts (CAFs) are important in tumor microenvironment (TME) driven cancer progression. However, CAFs are heterogeneous and still largely underdefined, better understanding their origins will identify new therapeutic strategies for cancer. Here, the authors discovered a new role of macrophage-myofibroblast transition (MMT) in cancer for de novo generating protumoral CAFs by resolving the transcriptome dynamics of tumor-associated macrophages (TAM) with single-cell resolution. MMT cells (MMTs) are observed in non-small-cell lung carcinoma (NSCLC) associated with CAF abundance and patient mortality. By fate-mapping study, RNA velocity, and pseudotime analysis, existence of novel macrophage-lineage-derived CAF subset in the TME of Lewis lung carcinoma (LLC) model is confirmed, which is directly transited via MMT from M2-TAM in vivo and bone-marrow-derived macrophages (BMDM) in vitro. Adoptive transfer of BMDM-derived MMTs markedly promote CAF formation in LLC-bearing mice. Mechanistically, a Smad3-centric regulatory network is upregulated in the MMTs of NSCLC, where chromatin immunoprecipitation sequencing(ChIP-seq) detects a significant enrichment of Smad3 binding on fibroblast differentiation genes in the macrophage-lineage cells in LLC-tumor. More importantly, macrophage-specific deletion and pharmaceutical inhibition of Smad3 effectively block MMT, therefore, suppressing the CAF formation and cancer progression in vivo. Thus, MMT may represent a novel therapeutic target of CAF for cancer immunotherapy.
Keywords: Smad3; cancer-associated fibroblasts; macrophage-myofibroblast transition; tumor microenvironment; tumor-associated macrophages.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Costa A., Kieffer Y., Scholer‐Dahirel A., Pelon F., Bourachot B., Cardon M., Sirven P., Magagna I., Fuhrmann L., Bernard C., Bonneau C., Kondratova M., Kuperstein I., Zinovyev A., Givel A. M., Parrini M. C., Soumelis V., Vincent‐Salomon A., Mechta‐Grigoriou F., Cancer Cell 2018, 33, 463. - PubMed
-
- Sakai T., Aokage K., Neri S., Nakamura H., Nomura S., Tane K., Miyoshi T., Sugano M., Kojima M., Fujii S., Kuwata T., Ochiai A., Iyoda A., Tsuboi M., Ishii G., Lung Cancer 2018, 126, 64. - PubMed
-
- Calaf G. M., Zepeda A. B., Castillo R. L., Figueroa C. A., Arias C., Figueroa E., Farias J. G., Int. J. Oncol. 2015, 47, 437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 14111720/Research Grants Council of Hong Kong
- 14111019/Research Grants Council of Hong Kong
- 14121816/Research Grants Council of Hong Kong
- 14117418/Research Grants Council of Hong Kong
- 14104019/Research Grants Council of Hong Kong
- C7018-16G/Research Grants Council of Hong Kong
- 14106518/Research Grants Council of Hong Kong
- ITS/068/18/Innovation and Technology Fund of Hong Kong
- 4620528/Faculty Innovation Award
- 4054386/Direct Grant for Research CUHK
- 4054440/Direct Grant for Research CUHK
- CARE program/Lui Che Woo Institute of Innovative Medicine
- 2019B121205005/The Guangdong-Hong Kong-Macao-Joint Labs Program from Guangdong Science and Technology
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
