Hydrogen sulfide blocks HIV rebound by maintaining mitochondrial bioenergetics and redox homeostasis
- PMID: 34792020
- PMCID: PMC8660018
- DOI: 10.7554/eLife.68487
Hydrogen sulfide blocks HIV rebound by maintaining mitochondrial bioenergetics and redox homeostasis
Abstract
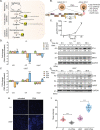
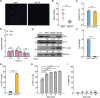
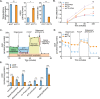
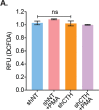
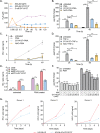
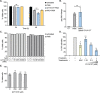
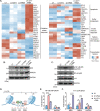
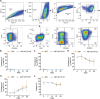
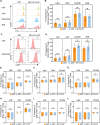
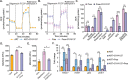
A fundamental challenge in human immunodeficiency virus (HIV) eradication is to understand how the virus establishes latency, maintains stable cellular reservoirs, and promotes rebound upon interruption of antiretroviral therapy (ART). Here, we discovered an unexpected role of the ubiquitous gasotransmitter hydrogen sulfide (H2S) in HIV latency and reactivation. We show that reactivation of HIV is associated with downregulation of the key H2S producing enzyme cystathionine-γ-lyase (CTH) and reduction in endogenous H2S. Genetic silencing of CTH disrupts redox homeostasis, impairs mitochondrial function, and remodels the transcriptome of latent cells to trigger HIV reactivation. Chemical complementation of CTH activity using a slow-releasing H2S donor, GYY4137, suppressed HIV reactivation and diminished virus replication. Mechanistically, GYY4137 blocked HIV reactivation by inducing the Keap1-Nrf2 pathway, inhibiting NF-κB, and recruiting the epigenetic silencer, YY1, to the HIV promoter. In latently infected CD4+ T cells from ART-suppressed human subjects, GYY4137 in combination with ART prevented viral rebound and improved mitochondrial bioenergetics. Moreover, prolonged exposure to GYY4137 exhibited no adverse influence on proviral content or CD4+ T cell subsets, indicating that diminished viral rebound is due to a loss of transcription rather than a selective loss of infected cells. In summary, this work provides mechanistic insight into H2S-mediated suppression of viral rebound and suggests exploration of H2S donors to maintain HIV in a latent form.
Keywords: GYY4137; T-cells; biochemistry; chemical biology; cystathionine-gamma-lyase; gasotransmitter; infectious disease; latency; microbiology; redox; viruses.
© 2021, Pal et al.
Conflict of interest statement
VP, RA, SR, PS, DM, AV, AS No competing interests declared
Figures




Similar articles
-
Hydrogen sulfide (H2S) coordinates redox balance, carbon metabolism, and mitochondrial bioenergetics to suppress SARS-CoV-2 infection.PLoS Pathog. 2025 May 19;21(5):e1013164. doi: 10.1371/journal.ppat.1013164. eCollection 2025 May. PLoS Pathog. 2025. PMID: 40388397 Free PMC article.
-
Role of hydrogen sulfide in paramyxovirus infections.J Virol. 2015 May;89(10):5557-68. doi: 10.1128/JVI.00264-15. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740991 Free PMC article.
-
Hydrogen Sulfide Is an Antiviral and Antiinflammatory Endogenous Gasotransmitter in the Airways. Role in Respiratory Syncytial Virus Infection.Am J Respir Cell Mol Biol. 2016 Nov;55(5):684-696. doi: 10.1165/rcmb.2015-0385OC. Am J Respir Cell Mol Biol. 2016. PMID: 27314446 Free PMC article.
-
Biological Effects of Morpholin-4-Ium 4 Methoxyphenyl (Morpholino) Phosphinodithioate and Other Phosphorothioate-Based Hydrogen Sulfide Donors.Antioxid Redox Signal. 2020 Jan 10;32(2):145-158. doi: 10.1089/ars.2019.7896. Antioxid Redox Signal. 2020. PMID: 31642346 Review.
-
Targeting hydrogen sulfide as a promising therapeutic strategy for atherosclerosis.Int J Cardiol. 2014 Mar 15;172(2):313-7. doi: 10.1016/j.ijcard.2014.01.068. Epub 2014 Jan 24. Int J Cardiol. 2014. PMID: 24491853 Review.
Cited by
-
Anomalous HIV-1 RNA, How Cap-Methylation Segregates Viral Transcripts by Form and Function.Viruses. 2022 Apr 29;14(5):935. doi: 10.3390/v14050935. Viruses. 2022. PMID: 35632676 Free PMC article. Review.
-
Hydrogen sulfide maintains mitochondrial homeostasis and regulates ganoderic acids biosynthesis by SQR under heat stress in Ganoderma lucidum.Redox Biol. 2024 Aug;74:103227. doi: 10.1016/j.redox.2024.103227. Epub 2024 Jun 6. Redox Biol. 2024. PMID: 38865903 Free PMC article.
-
GYY4137-Derived Hydrogen Sulfide Donates Electrons to the Mitochondrial Electron Transport Chain via Sulfide: Quinone Oxidoreductase in Endothelial Cells.Antioxidants (Basel). 2023 Feb 27;12(3):587. doi: 10.3390/antiox12030587. Antioxidants (Basel). 2023. PMID: 36978834 Free PMC article.
-
Ataxin-2: a powerful RNA-binding protein.Discov Oncol. 2024 Jul 22;15(1):298. doi: 10.1007/s12672-024-01158-y. Discov Oncol. 2024. PMID: 39039334 Free PMC article. Review.
-
GLUT1 overexpression in CAR-T cells induces metabolic reprogramming and enhances potency.Nat Commun. 2024 Oct 6;15(1):8658. doi: 10.1038/s41467-024-52666-y. Nat Commun. 2024. PMID: 39370422 Free PMC article.
References
-
- Alzahrani J, Hussain T, Simar D, Palchaudhuri R, Abdel-Mohsen M, Crowe SM, Mbogo GW, Palmer CS. Inflammatory and immunometabolic consequences of gut dysfunction in HIV: Parallels with IBD and implications for reservoir persistence and non-AIDS comorbidities. EBioMedicine. 2019;46:522–531. doi: 10.1016/j.ebiom.2019.07.027. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

