Effect of amino acid-based formula added to four-food elimination in adult eosinophilic esophagitis patients: A randomized clinical trial
- PMID: 34792264
- PMCID: PMC9286809
- DOI: 10.1111/nmo.14291
Effect of amino acid-based formula added to four-food elimination in adult eosinophilic esophagitis patients: A randomized clinical trial
Abstract
Background: Elimination of key foods restricts dietary options in eosinophilic esophagitis (EoE) patients. Addition of amino acid-based formula (AAF) to an elimination diet might facilitate adherence and, therefore, enhance efficacy of dietary management.
Aim: To evaluate whether addition of AAF to a four-food elimination diet (FFED) is more effective than FFED alone in decreasing eosinophilia, endoscopic signs, and clinical outcomes.
Methods: This randomized controlled trial enrolled 41 adult patients with active EoE (≥15 eosinophils (eos) per high power field (hpf)) at baseline biopsy. Subjects were randomized (1:1 ratio) to groups given a FFED or FFED with addition of AAF providing 30% of their daily energy needs (FFED + AAF). Histological disease activity, endoscopic signs, symptoms, and disease-related quality of life (EoEQoL) were measured at baseline and after 6 weeks of intervention.
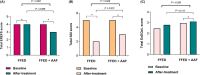
Results: Patients (60% male, age 34.5 (interquartile range (IQR) 29-42.8 years)) were randomized to FFED (n = 20) or FFED + AAF (n = 21); 40 participants completed the diet. Complete histological remission (<15 eos/hpf) was achieved in 48% of FFED + AAF subjects (n = 21) vs. 25% of FFED subjects (n = 20), respectively (p = 0.204). Peak eosinophil counts (PEC) decreased significantly in both groups between baseline and week 6, but the change in PEC between groups was not different (p = 0.130). A significant but similar endoscopic and symptomatic reduction was observed in both groups (all; p<0.05). Total EoEQoL scores significantly improved in the FFED + AAF group between baseline and week 6 (p = 0.007), and not in the FFED group.
Conclusion: The addition of AAF to a FFED did not lead to a larger decrease in PEC between baseline and 6 weeks, but may result in a significant improvement of QoL in adult EoE patients NL6014 (NTR6778).
Keywords: elemental diet; elimination diet; eosinophilic esophagitis; esophageal eosinophilia and allergy; quality of life.
© 2021 The Authors. Neurogastroenterology & Motility published by John Wiley & Sons Ltd.
Figures

References
-
- Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. sponsored by the american gastroenterological association (AGA) Institute and North American society of pediatric gastroenterol. Gastroenterology. 2007;133:1342‐1363. - PubMed
-
- Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3‐20. - PubMed
-
- Attwood S, Smyrk T, Demeester T, Jones J. Eosophageal eosinophilia with dysphagia. a distinct clincopathological syndrome. J Dig Dis. 1993;38:109‐116. - PubMed
-
- Navarro P, Arias Á, Arias‐González L, Laserna‐Mendieta EJ, Ruiz‐Ponce M, Lucendo AJ, et al. Systematic review with meta‐analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population‐based studies. Aliment Pharmacol Ther. 2019;49:1116‐1125. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical

