What Is the Role of the Environment in the Emergence of Novel Antibiotic Resistance Genes? A Modeling Approach
- PMID: 34792330
- PMCID: PMC8655980
- DOI: 10.1021/acs.est.1c02977
What Is the Role of the Environment in the Emergence of Novel Antibiotic Resistance Genes? A Modeling Approach
Abstract
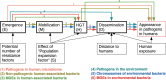
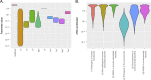
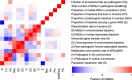
It is generally accepted that intervention strategies to curb antibiotic resistance cannot solely focus on human and veterinary medicine but must also consider environmental settings. While the environment clearly has a role in transmission of resistant bacteria, its role in the emergence of novel antibiotic resistance genes (ARGs) is less clear. It has been suggested that the environment constitutes an enormous recruitment ground for ARGs to pathogens, but its extent is practically unknown. We have constructed a model framework for resistance emergence and used available quantitative data on relevant processes to identify limiting steps in the appearance of ARGs in human pathogens. We found that in a majority of possible scenarios, the environment would only play a minor role in the emergence of novel ARGs. However, the uncertainty is enormous, highlighting an urgent need for more quantitative data. Specifically, more data is most needed on the fitness costs of ARG carriage, the degree of dispersal of resistant bacteria from the environment to humans, and the rates of mobilization and horizontal transfer of ARGs. This type of data is instrumental to determine which processes should be targeted for interventions to curb development and transmission of ARGs in the environment.
Keywords: human and animal health; mobile genetic elements; mobilization; origin of antibiotic resistance genes; pathogenic bacteria.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
The Association between Insertion Sequences and Antibiotic Resistance Genes.mSphere. 2020 Sep 2;5(5):e00418-20. doi: 10.1128/mSphere.00418-20. mSphere. 2020. PMID: 32878926 Free PMC article.
-
Anthropogenic disturbances on antibiotic resistome along the Yarlung Tsangpo River on the Tibetan Plateau: Ecological dissemination mechanisms of antibiotic resistance genes to bacterial pathogens.Water Res. 2021 Sep 1;202:117447. doi: 10.1016/j.watres.2021.117447. Epub 2021 Jul 20. Water Res. 2021. PMID: 34325101
-
Whole Genome Analysis of 335 New Bacterial Species from Human Microbiota Reveals a Huge Reservoir of Transferable Antibiotic Resistance Determinants.Int J Mol Sci. 2022 Feb 15;23(4):2137. doi: 10.3390/ijms23042137. Int J Mol Sci. 2022. PMID: 35216256 Free PMC article.
-
Research progress on distribution, migration, transformation of antibiotics and antibiotic resistance genes (ARGs) in aquatic environment.Crit Rev Biotechnol. 2018 Dec;38(8):1195-1208. doi: 10.1080/07388551.2018.1471038. Epub 2018 May 28. Crit Rev Biotechnol. 2018. PMID: 29807455 Review.
-
Enrichment of antibiotic resistance genes (ARGs) in polyaromatic hydrocarbon-contaminated soils: a major challenge for environmental health.Environ Sci Pollut Res Int. 2021 Mar;28(10):12178-12189. doi: 10.1007/s11356-020-12171-3. Epub 2021 Jan 4. Environ Sci Pollut Res Int. 2021. PMID: 33394421 Review.
Cited by
-
Uncovering the diverse hosts of tigecycline resistance gene tet(X4) in anaerobic digestion systems treating swine manure by epicPCR.Water Res X. 2023 Mar 1;19:100174. doi: 10.1016/j.wroa.2023.100174. eCollection 2023 May 1. Water Res X. 2023. PMID: 36915394 Free PMC article.
-
Genome-centric analyses of 165 metagenomes show that mobile genetic elements are crucial for the transmission of antimicrobial resistance genes to pathogens in activated sludge and wastewater.Microbiol Spectr. 2024 Mar 5;12(3):e0291823. doi: 10.1128/spectrum.02918-23. Epub 2024 Jan 30. Microbiol Spectr. 2024. PMID: 38289113 Free PMC article.
-
Epidemiological Prevalence of Phenotypical Resistances and Mobilised Colistin Resistance in Avian Commensal and Pathogenic E. coli from Denmark, France, The Netherlands, and the UK.Antibiotics (Basel). 2022 May 7;11(5):631. doi: 10.3390/antibiotics11050631. Antibiotics (Basel). 2022. PMID: 35625275 Free PMC article.
-
Population Ecology-Quantitative Microbial Risk Assessment (QMRA) Model for Antibiotic-Resistant and Susceptible E. coli in Recreational Water.Environ Sci Technol. 2025 Mar 11;59(9):4266-4281. doi: 10.1021/acs.est.4c07248. Epub 2025 Feb 26. Environ Sci Technol. 2025. PMID: 40008406
-
Occurrence of selected Covid-19 drugs in surface water resources: a review of their sources, pathways, receptors, fate, ecotoxicity, and possible interactions with heavy metals in aquatic ecosystems.Environ Geochem Health. 2024 Nov 28;47(1):3. doi: 10.1007/s10653-024-02293-9. Environ Geochem Health. 2024. PMID: 39607624 Free PMC article. Review.
References
-
- O’Neill J.Tackling Drug-resistant Infections Globally: Final Report and Recommendations–The Review on Antimicrobial Resistance; Wellcome Trust and HM Government, 2016.
-
- European Commission . A European One Health Action Plan against Antimicrobial Resistance (AMR); European Commission, 2017.
-
- Ashbolt N. J.; Amézquita A.; Backhaus T.; Borriello P.; Brandt K. K.; Collignon P.; Coors A.; Finley R.; Gaze W. H.; Heberer T.; Lawrence J. R.; Larsson D. G. J.; McEwen S. A.; Ryan J. J.; Schönfeld J.; Silley P.; Snape J. R.; Van den Eede C.; Topp E. Human Health Risk Assessment (HHRA) for Environmental Development and Transfer of Antibiotic Resistance. Environ. Health Perspect. 2013, 121, 993–1001. 10.1289/ehp.1206316. - DOI - PMC - PubMed
-
- Gaze W. H.; Krone S. M.; Larsson D. G. J.; Li X.-Z.; Robinson J. A.; Simonet P.; Smalla K.; Timinouni M.; Topp E.; Wellington E. M.; Wright G. D.; Zhu Y.-G. Influence of Humans on Evolution and Mobilization of Environmental Antibiotic Resistome. Emerging Infect. Dis. 2013, 19, e12087110.3201/eid1907.120871. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

