Comparing immunogenicity and protective efficacy of the yellow fever 17D vaccine in mice
- PMID: 34792431
- PMCID: PMC8648041
- DOI: 10.1080/22221751.2021.2008772
Comparing immunogenicity and protective efficacy of the yellow fever 17D vaccine in mice
Abstract
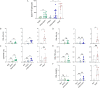
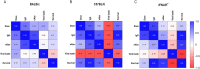
The live-attenuated yellow fever 17D (YF17D) vaccine is one of the most efficacious human vaccines and also employed as a vector for novel vaccines. However, in the lack of appropriate immunocompetent small animal models, mechanistic insight in YF17D-induced protective immunity remains limited. To better understand YF17D vaccination and to identify a suitable mouse model, we evaluated the immunogenicity and protective efficacy of YF17D in five complementary mouse models, i.e. wild-type (WT) BALB/c, C57BL/6, IFN-α/β receptor (IFNAR-/-) deficient mice, and in WT mice in which type I IFN signalling was temporally ablated by an IFNAR blocking (MAR-1) antibody. Alike in IFNAR-/- mice, YF17D induced in either WT mice strong humoral immune responses dominated by IgG2a/c isotype (Th1 type) antibodies, yet only when IFNAR was blocked. Vigorous cellular immunity characterized by CD4+ T-cells producing IFN-γ and TNF-α were mounted in MAR-1 treated C57BL/6 and in IFNAR-/- mice. Surprisingly, vaccine-induced protection was largely mouse model dependent. Full protection against lethal intracranial challenge and a massive reduction of virus loads was conferred already by a minimal dose of 2 PFU YF17D in BALB/c and IFNAR-/- mice, but not in C57BL/6 mice. Correlation analysis of infection outcome with pre-challenge immunological markers indicates that YFV-specific IgG might suffice for protection, even in the absence of detectable levels of neutralizing antibodies. Finally, we propose that, in addition to IFNAR-/- mice, C57BL/6 mice with temporally blocked IFN-α/β receptors represent a promising immunocompetent mouse model for the study of YF17D-induced immunity and evaluation of YF17D-derived vaccines.
Keywords: Yellow fever 17D; correlate of protection; live-attenuated vaccine; mouse models; type I IFN response.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- WHO . Fact Sheet: Yellow fever. WHO Fact Sheet. 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
