Pan-genome analysis identifies intersecting roles for Pseudomonas specialized metabolites in potato pathogen inhibition
- PMID: 34792466
- PMCID: PMC8719888
- DOI: 10.7554/eLife.71900
Pan-genome analysis identifies intersecting roles for Pseudomonas specialized metabolites in potato pathogen inhibition
Abstract
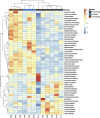
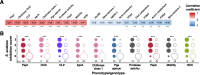
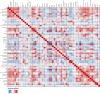
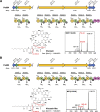
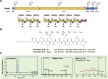
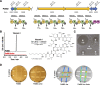
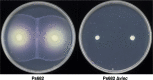
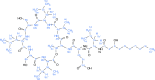
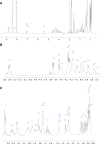
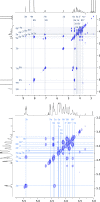
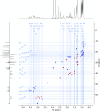
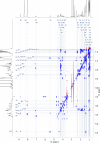
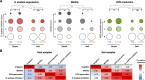
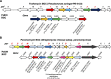
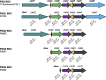
Agricultural soil harbors a diverse microbiome that can form beneficial relationships with plants, including the inhibition of plant pathogens. Pseudomonas spp. are one of the most abundant bacterial genera in the soil and rhizosphere and play important roles in promoting plant health. However, the genetic determinants of this beneficial activity are only partially understood. Here, we genetically and phenotypically characterize the Pseudomonas fluorescens population in a commercial potato field, where we identify strong correlations between specialized metabolite biosynthesis and antagonism of the potato pathogens Streptomyces scabies and Phytophthora infestans. Genetic and chemical analyses identified hydrogen cyanide and cyclic lipopeptides as key specialized metabolites associated with S. scabies inhibition, which was supported by in planta biocontrol experiments. We show that a single potato field contains a hugely diverse and dynamic population of Pseudomonas bacteria, whose capacity to produce specialized metabolites is shaped both by plant colonization and defined environmental inputs.
Keywords: Pseudomonas; Streptomyces scabies; biocontrol; genetics; genomics; infectious disease; microbiology; natural products; plant disease; potato; pseudomonas fluorescens.
Plain language summary
Potato scab and blight are two major diseases which can cause heavy crop losses. They are caused, respectively, by the bacterium Streptomyces scabies and an oomycete (a fungus-like organism) known as Phytophthora infestans. Fighting these disease-causing microorganisms can involve crop management techniques – for example, ensuring that a field is well irrigated helps to keep S. scabies at bay. Harnessing biological control agents can also offer ways to control disease while respecting the environment. Biocontrol bacteria, such as Pseudomonas, can produce compounds that keep S. scabies and P. infestans in check. However, the identity of these molecules and how irrigation can influence Pseudomonas population remains unknown. To examine these questions, Pacheco-Moreno et al. sampled and isolated hundreds of Pseudomonas strains from a commercial potato field, closely examining the genomes of 69 of these. Comparing the genetic information of strains based on whether they could control the growth of S. scabies revealed that compounds known as cyclic lipopeptides are key to controlling the growth of S. scabies and P. infestans. Whether the field was irrigated also had a large impact on the strains forming the Pseudomonas population. Working out how Pseudomonas bacteria block disease could speed up the search for biological control agents. The approach developed by Pacheco-Moreno et al. could help to predict which strains might be most effective based on their genetic features. Similar experiments could also work for other combinations of plants and diseases.
© 2021, Pacheco-Moreno et al.
Conflict of interest statement
AP, FS, JF, CT, LG, RD, NK, AK, LA, SN, KF, JC, MT, GC, JM, AT No competing interests declared, GT affiliated with VCS Potatoes and has no financial interests to declare
Figures







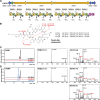
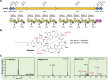
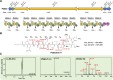
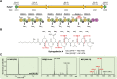













References
-
- Alsohim AS, Taylor TB, Barrett GA, Gallie J, Zhang XX, Altamirano-Junqueira AE, Johnson LJ, Rainey PB, Jackson RW. The biosurfactant viscosin produced by Pseudomonas fluorescens SBW25 aids spreading motility and plant growth promotion. Environmental Microbiology. 2014;16:2267–2281. doi: 10.1111/1462-2920.12469. - DOI - PubMed
-
- Andrade M, Niederheitmann M, de Paula Ribeiro SRR, Oliveira LC, Pozza EA, Pinto C. Development and validation of a standard area diagram to assess common scab in potato tubers. European Journal of Plant Pathology. 2019;154:739–750. doi: 10.1007/s10658-019-01697-z. - DOI
-
- Aron AT, Gentry EC, McPhail KL, Nothias LF, Nothias-Esposito M, Bouslimani A, Petras D, Gauglitz JM, Sikora N, Vargas F, van der Hooft JJJ, Ernst M, Kang KB, Aceves CM, Caraballo-Rodríguez AM, Koester I, Weldon KC, Bertrand S, Roullier C, Sun K, Tehan RM, Boya P CA, Christian MH, Gutiérrez M, Ulloa AM, Tejeda Mora JA, Mojica-Flores R, Lakey-Beitia J, Vásquez-Chaves V, Zhang Y, Calderón AI, Tayler N, Keyzers RA, Tugizimana F, Ndlovu N, Aksenov AA, Jarmusch AK, Schmid R, Truman AW, Bandeira N, Wang M, Dorrestein PC. Reproducible molecular networking of untargeted mass spectrometry data using GNPS. Nature Protocols. 2020;15:1954–1991. doi: 10.1038/s41596-020-0317-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- BB/J004553/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/J/000PR9790/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J004596/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/J/000PR9797/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/M011216/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

