Cryo-EM structure of human GPR158 receptor coupled to the RGS7-Gβ5 signaling complex
- PMID: 34793198
- PMCID: PMC8926151
- DOI: 10.1126/science.abl4732
Cryo-EM structure of human GPR158 receptor coupled to the RGS7-Gβ5 signaling complex
Abstract
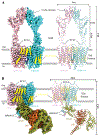
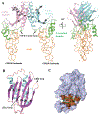
GPR158 is an orphan G protein–coupled receptor (GPCR) highly expressed in the brain, where it controls synapse formation and function. GPR158 has also been implicated in depression, carcinogenesis, and cognition. However, the structural organization and signaling mechanisms of GPR158 are largely unknown. We used single-particle cryo–electron microscopy (cryo-EM) to determine the structures of human GPR158 alone and bound to an RGS signaling complex. The structures reveal a homodimeric organization stabilized by a pair of phospholipids and the presence of an extracellular Cache domain, an unusual ligand-binding domain in GPCRs. We further demonstrate the structural basis of GPR158 coupling to RGS7-Gβ5. Together, these results provide insights into the unusual biology of orphan receptors and the formation of GPCR-RGS complexes.
Conflict of interest statement
Figures

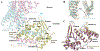
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

