Donor Clonal Hematopoiesis and Recipient Outcomes After Transplantation
- PMID: 34793200
- PMCID: PMC8718176
- DOI: 10.1200/JCO.21.02286
Donor Clonal Hematopoiesis and Recipient Outcomes After Transplantation
Abstract
Purpose: Clonal hematopoiesis (CH) can be transmitted from a donor to a recipient during allogeneic hematopoietic cell transplantation. Exclusion of candidate donors with CH is controversial since its impact on recipient outcomes and graft alloimmune function is uncertain.
Patients and methods: We performed targeted error-corrected sequencing on samples from 1,727 donors age 40 years or older and assessed the effect of donor CH on recipient clinical outcomes. We measured long-term engraftment of 102 donor clones and cytokine levels in 256 recipients at 3 and 12 months after transplant.
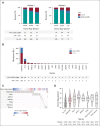
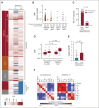
Results: CH was present in 22.5% of donors, with DNMT3A (14.6%) and TET2 (5.2%) mutations being most common; 85% of donor clones showed long-term engraftment in recipients after transplantation, including clones with a variant allele fraction < 0.01. DNMT3A-CH with a variant allele fraction ≥ 0.01, but not smaller clones, was associated with improved recipient overall (hazard ratio [HR], 0.79; P = .042) and progression-free survival (HR, 0.72; P = .003) after adjustment for significant clinical variables. In patients who received calcineurin-based graft-versus-host disease prophylaxis, donor DNMT3A-CH was associated with reduced relapse (subdistribution HR, 0.59; P = .014), increased chronic graft-versus-host disease (subdistribution HR, 1.36; P = .042), and higher interleukin-12p70 levels in recipients. No recipient of sole DNMT3A or TET2-CH developed donor cell leukemia (DCL). In seven of eight cases, DCL evolved from donor CH with rare TP53 or splicing factor mutations or from donors carrying germline DDX41 mutations.
Conclusion: Donor CH is closely associated with clinical outcomes in transplant recipients, with differential impact on graft alloimmune function and potential for leukemic transformation related to mutated gene and somatic clonal abundance. Donor DNMT3A-CH is associated with improved recipient survival because of reduced relapse risk and with an augmented network of inflammatory cytokines in recipients. Risk of DCL in allogeneic hematopoietic cell transplantation is driven by somatic myelodysplastic syndrome-associated mutations or germline predisposition in donors.
Conflict of interest statement
Figures


References
-
- Schlenk RF, Döhner K, Krauter J, et al. : Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med 358:1909-1918, 2008 - PubMed
-
- Nakamura R, Saber W, Martens MJ, et al. : A multi-center biologic assignment trial comparing reduced intensity allogeneic hematopoietic cell transplantation to hypomethylating therapy or best supportive care in patients aged 50-75 with advanced myelodysplastic syndrome: Blood and Marrow Transplant Clinical Trials Network study 1102. Blood 136:19-21, 2020
-
- Be The Match : Age Requirements and Limits for Donating Bone Marrow. https://bethematch.org/transplant-basics/matching-patients-with-donors/w...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

