Inulin-grown Faecalibacterium prausnitzii cross-feeds fructose to the human intestinal epithelium
- PMID: 34793284
- PMCID: PMC8604389
- DOI: 10.1080/19490976.2021.1993582
Inulin-grown Faecalibacterium prausnitzii cross-feeds fructose to the human intestinal epithelium
Abstract
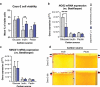
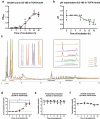
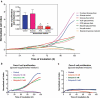
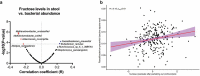
Many chronic diseases are associated with decreased abundance of the gut commensal Faecalibacterium prausnitzii. This strict anaerobe can grow on dietary fibers, e.g., prebiotics, and produce high levels of butyrate, often associated to epithelial metabolism and health. However, little is known about other F. prausnitzii metabolites that may affect the colonic epithelium. Here, we analyzed prebiotic cross-feeding between F. prausnitzii and intestinal epithelial (Caco-2) cells in a "Human-oxygen Bacteria-anaerobic" coculture system. Inulin-grown F. prausnitzii enhanced Caco-2 viability and suppressed inflammation- and oxidative stress-marker expression. Inulin-grown F. prausnitzii produced excess butyrate and fructose, but only fructose efficiently promoted Caco-2 growth. Finally, fecal microbial taxonomy analysis (16S sequencing) from healthy volunteers (n = 255) showed the strongest positive correlation for F. prausnitzii abundance and stool fructose levels. We show that fructose, produced and accumulated in a fiber-rich colonic environment, supports colonic epithelium growth, while butyrate does not.
Keywords: Faecalibacterium; Gut bacteria; dysbiosis; fructose; intestinal epithelium; inulin.
Conflict of interest statement
RKW acted as consultant for Takeda and received unrestricted research grants from Takeda. RKW received unrestricted research grants from Johnson & Johnson Pharmaceuticals, Tramedico and Ferring, and received speaker fees from AbbVie, MSD, Olympus and AstraZeneca. GD received research grant from Royal DSM and speaker’s fees from Janssen Pharmaceuticals, Pfizer and Abbvie. All other authors have no conflicts of interest to declare.
Figures


References
-
- Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. Internet] 2015. [cited 2016 Nov 3]; 12:205–18. Available from(4)http://www.nature.com/doifinder/10.1038/nrgastro.2015.34 - DOI - PubMed
-
- Eckburg PB. Diversity of the human intestinal microbial flora. Science. Internet] 2005. [cited 2018 Apr 23]; 308(5728):1635–1638. Available from http://www.sciencemag.org/cgi/doi/10.1126/science.1110591 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
