SIRT6 protects vascular smooth muscle cells from osteogenic transdifferentiation via Runx2 in chronic kidney disease
- PMID: 34793336
- PMCID: PMC8718147
- DOI: 10.1172/JCI150051
SIRT6 protects vascular smooth muscle cells from osteogenic transdifferentiation via Runx2 in chronic kidney disease
Abstract
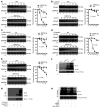
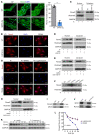
Vascular calcification (VC) is regarded as an important pathological change lacking effective treatment and associated with high mortality. Sirtuin 6 (SIRT6) is a member of the Sirtuin family, a class III histone deacetylase and a key epigenetic regulator. SIRT6 has a protective role in patients with chronic kidney disease (CKD). However, the exact role and molecular mechanism of SIRT6 in VC in patients with CKD remain unclear. Here, we demonstrated that SIRT6 was markedly downregulated in peripheral blood mononuclear cells (PBMCs) and in the radial artery tissue of patients with CKD with VC. SIRT6-transgenic (SIRT6-Tg) mice showed alleviated VC, while vascular smooth muscle cell-specific (VSMC-specific) SIRT6 knocked-down mice showed severe VC in CKD. SIRT6 suppressed the osteogenic transdifferentiation of VSMCs via regulation of runt-related transcription factor 2 (Runx2). Coimmunoprecipitation (co-IP) and immunoprecipitation (IP) assays confirmed that SIRT6 bound to Runx2. Moreover, Runx2 was deacetylated by SIRT6 and further promoted nuclear export via exportin 1 (XPO1), which in turn caused degradation of Runx2 through the ubiquitin-proteasome system. These results demonstrated that SIRT6 prevented VC by suppressing the osteogenic transdifferentiation of VSMCs, and as such targeting SIRT6 may be an appealing therapeutic target for VC in CKD.
Keywords: Cardiovascular disease; Cell Biology; Chronic kidney disease; Ubiquitin-proteosome system; Vascular Biology.
Figures





Comment in
-
A molecular target of vascular calcification in chronic kidney disease.J Clin Invest. 2022 Jan 4;132(1):e156257. doi: 10.1172/JCI156257. J Clin Invest. 2022. PMID: 34981783 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

