Multistate Outbreak of SARS-CoV-2 Infections, Including Vaccine Breakthrough Infections, Associated with Large Public Gatherings, United States
- PMID: 34793690
- PMCID: PMC8714214
- DOI: 10.3201/eid2801.212220
Multistate Outbreak of SARS-CoV-2 Infections, Including Vaccine Breakthrough Infections, Associated with Large Public Gatherings, United States
Abstract
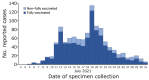
During July 2021, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.1.617.2 variant infections, including vaccine breakthrough infections, occurred after large public gatherings in Provincetown, Massachusetts, USA, prompting a multistate investigation. Public health departments identified primary and secondary cases by using coronavirus disease surveillance data, case investigations, and contact tracing. A primary case was defined as SARS-CoV-2 detected <14 days after travel to or residence in Provincetown during July 3-17. A secondary case was defined as SARS-CoV-2 detected <14 days after close contact with a person who had a primary case but without travel to or residence in Provincetown during July 3-August 10. We identified 1,098 primary cases and 30 secondary cases associated with 26 primary cases among fully and non-fully vaccinated persons. Large gatherings can have widespread effects on SARS-CoV-2 transmission, and fully vaccinated persons should take precautions, such as masking, to prevent SARS-CoV-2 transmission, particularly during substantial or high transmission.
Keywords: COVID-19; COVID-19 vaccines; Massachusetts; SARS-CoV-2; United States; breakthrough infections; coronavirus disease; coronaviruses; disease outbreaks; multistate outbreak; respiratory infections; severe acute respiratory syndrome coronavirus 2; viruses; zoonoses.
Figures


References
-
- Centers for Disease Control and Prevention. COVID data tracker: variant proportions, August 2021. [cited 2021 Nov 6]. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
-
- Tenforde MW, Patel MM, Ginde AA, Douin DJ, Talbot HK, Casey JD, et al.; Influenza and Other Viruses in the Acutely Ill (IVY) Network. Effectiveness of SARS-CoV-2 mRNA vaccines for preventing COVID-19 hospitalizations in the United States. Clin Infect Dis. 2021;•••:ciab687; Epub ahead of print. 10.1093/cid/ciab687 - DOI - PMC - PubMed
-
- Fowlkes A, Gaglani M, Groover K, Thiese MS, Tyner H, Ellingson K; HEROES-RECOVER Cohorts. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection among frontline workers before and during B.1.617.2 (Delta) variant predominance—eight U.S. locations, December 2020‒August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1167–9. 10.15585/mmwr.mm7034e4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

