GABAkines - Advances in the discovery, development, and commercialization of positive allosteric modulators of GABAA receptors
- PMID: 34793859
- PMCID: PMC9787737
- DOI: 10.1016/j.pharmthera.2021.108035
GABAkines - Advances in the discovery, development, and commercialization of positive allosteric modulators of GABAA receptors
Abstract
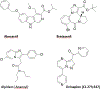
Positive allosteric modulators of γ-aminobutyric acid-A (GABAA) receptors or GABAkines have been widely used medicines for over 70 years for anxiety, epilepsy, sleep, and other disorders. Traditional GABAkines like diazepam have safety and tolerability concerns that include sedation, motor-impairment, respiratory depression, tolerance and dependence. Multiple GABAkines have entered clinical development but the issue of side-effects has not been fully solved. The compounds that are presently being developed and commercialized include several neuroactive steroids (an allopregnanolone formulation (brexanolone), an allopregnanolone prodrug (LYT-300), Sage-324, zuranolone, and ganaxolone), the α2/3-preferring GABAkine, KRM-II-81, and the α2/3/5-preferring GABAkine PF-06372865 (darigabat). The neuroactive steroids are in clinical development for post-partum depression, intractable epilepsy, tremor, status epilepticus, and genetic epilepsy disorders. Darigabat is in development for epilepsy and anxiety. The imidazodiazepine, KRM-II-81 is efficacious in animal models for the treatment of epilepsy and post-traumatic epilepsy, acute and chronic pain, as well as anxiety and depression. The efficacy of KRM-II-81 in models of pharmacoresistant epilepsy, preventing the development of seizure sensitization, and in brain tissue of intractable epileptic patients bodes well for improved therapeutics. Medicinal chemistry efforts are also ongoing to identify novel and improved GABAkines. The data document gaps in our understanding of the molecular pharmacology of GABAkines that drive differential pharmacological profiles, but emphasize advancements in the ability to successfully utilize GABAA receptor potentiation for therapeutic gain in neurology and psychiatry.
Keywords: Anxiety; Darigabat; Depression; Epilepsy; GABAkines; KRM-II-81; Neuroactive steroids; Pain.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest James Cook and Michael Poe are named as inventors on patents describing KRM-II-81 and analogs, certain rights to which have been licensed to RespireRx Pharmaceuticals Inc. Rok Cerne, Michael Poe, James Cook, and Jeffrey Witkin are members of the research advisory group for RespireRx Pharmaceuticals Inc. and Arnold Lippa serves as Executive Chairman and Chief Scientific Officer.
Figures
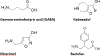



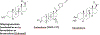

References
-
- Adelson PD (2001) Temporal lobectomy in children with intractable seizures. Pediatr Neurosurg 34:268–277. - PubMed
-
- Aden GC, Thein SG (1980) Alprazolam compared to diazepam and placebo in the treatment of anxiety. J Clin Psychiatry 41:245–248. - PubMed
-
- Afzalimoghaddam M, Khademi MF, Mirfazaelian H, Payandemehr P, Karimialavijeh E, Jalali A (2021) Comparing Diazepam Plus Fentanyl With Midazolam Plus Fentanyl in the Moderate Procedural Sedation of Anterior Shoulder Dislocations: A Randomized Clinical Trial. J Emerg Med 60:1–7. - PubMed
-
- Ahwazi HH, Abdijadid S (2021) Chlordiazepoxide. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Available at: http://www.ncbi.nlm.nih.gov/books/NBK547659/ [Accessed April 20, 2021].
-
- Alt A, Nisenbaum ES, Bleakman D, Witkin JM (2006) A role for AMPA receptors in mood disorders. Biochem Pharmacol 71:1273–1288. - PubMed

