Efanesoctocog alfa for hemophilia A: results from a phase 1 repeat-dose study
- PMID: 34794179
- PMCID: PMC8864644
- DOI: 10.1182/bloodadvances.2021006119
Efanesoctocog alfa for hemophilia A: results from a phase 1 repeat-dose study
Erratum in
-
Lissitchkov T, Willemze A, Katragadda S, et al. Efanesoctocog alfa for hemophilia A: results from a phase 1 repeat-dose study. Blood Adv. 2022;6(4):1089-1094.Blood Adv. 2022 Jun 28;6(12):3625. doi: 10.1182/bloodadvances.2022007724. Blood Adv. 2022. PMID: 35723890 Free PMC article. Clinical Trial. No abstract available.
Abstract
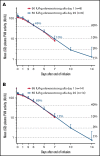
Efanesoctocog alfa (rFVIIIFc-VWF-XTEN; BIVV001) is a new class of factor VIII (FVIII) replacement that breaks the von Willebrand factor-imposed FVIII half-life ceiling. In a phase 1/2a study, single-dose efanesoctocog alfa was well tolerated, and no safety concerns were identified. We evaluated the safety, tolerability, and pharmacokinetics of repeat-dose efanesoctocog alfa in a phase 1 study in previously treated adults (≥150 exposure days) with severe hemophilia A. Participants received 4 once weekly doses of efanesoctocog alfa (cohort 1, 50 IU/kg; cohort 2, 65 IU/kg). All enrolled participants (cohort 1, n = 10; cohort 2, n = 14) completed the study. Inhibitor development to FVIII was not detected. After the last dose of efanesoctocog alfa, geometric mean (range) FVIII activity half-life, area under the activity-time curve, and steady-state maximum concentration for cohort 1 and cohort 2 were 41.3 (34.2-50.1) and 37.3 (28.9-43.8) hours, 8290 (5810-10 300) and 11 200 (7040-15 800) hours × IU/dL, and 131 (96-191) and 171 (118-211) IU/dL, respectively. There was minimal accumulation after 4 doses. Mean FVIII activity for cohort 1 and cohort 2, respectively, was 46% and 69% on day 3 postdose and 10% and 12% on day 7 postdose. Overall, 4 once-weekly doses of efanesoctocog alfa were well tolerated, no safety concerns were identified, and no bleeds were reported during the treatment period. Once-weekly efanesoctocog alfa provided high sustained FVIII activity within the normal to near-normal range for 3 to 4 days postdose and may improve protection against bleeds in patients with hemophilia A. The trial is study 2018-001535-51 in the EU Clinical Trials Register.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures


References
-
- Ahnström J, Berntorp E, Lindvall K, Björkman S. A 6-year follow-up of dosing, coagulation factor levels and bleedings in relation to joint status in the prophylactic treatment of haemophilia. Haemophilia. 2004;10(6):689-697. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

