Tisagenlecleucel outcomes in relapsed/refractory extramedullary ALL: a Pediatric Real World CAR Consortium Report
- PMID: 34794180
- PMCID: PMC8791593
- DOI: 10.1182/bloodadvances.2021005564
Tisagenlecleucel outcomes in relapsed/refractory extramedullary ALL: a Pediatric Real World CAR Consortium Report
Abstract
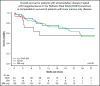
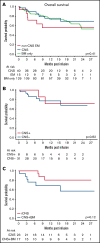
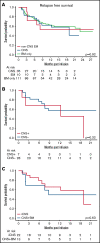
Chimeric antigen receptor (CAR) T cells have transformed the therapeutic options for relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia. Data for CAR therapy in extramedullary (EM) involvement are limited. Retrospective data were abstracted from the Pediatric Real World CAR Consortium (PRWCC) of 184 infused patients from 15 US institutions. Response (complete response) rate, overall survival (OS), relapse-free survival (RFS), and duration of B-cell aplasia (BCA) in patients referred for tisagenlecleucel with EM disease (both central nervous system (CNS)3 and non-CNS EM) were compared with bone marrow (BM) only. Patients with CNS disease were further stratified for comparison. Outcomes are reported on 55 patients with EM disease before CAR therapy (CNS3, n = 40; non-CNS EM, n = 15). The median age at infusion in the CNS cohort was 10 years (range, <1-25 years), and in the non-CNS EM cohort it was 13 years (range, 2-26 years). In patients with CNS disease, 88% (35 of 40) achieved a complete response vs only 66% (10 of 15) with non-CNS EM disease. Patients with CNS disease (both with and without BM involvement) had 24-month OS outcomes comparable to those of non-CNS EM or BM only (P = .41). There was no difference in 12-month RFS between CNS, non-CNS EM, or BM-only patients (P = .92). No increased toxicity was seen with CNS or non-CNS EM disease (P = .3). Active CNS disease at time of infusion did not affect outcomes. Isolated CNS disease trended toward improved OS compared with combined CNS and BM (P = .12). R/R EM disease can be effectively treated with tisagenlecleucel; toxicity, relapse, and survival rates are comparable to those of patients with BM-only disease. Outcomes for isolated CNS relapse are encouraging.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. - PubMed
-
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541-1552. - PubMed
-
- Gaynon PS. Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol. 2005;131(5):579-587. - PubMed

