Prescription opioid treatment for non-cancer pain and initiation of injection drug use: large retrospective cohort study
- PMID: 34794949
- PMCID: PMC8600402
- DOI: 10.1136/bmj-2021-066965
Prescription opioid treatment for non-cancer pain and initiation of injection drug use: large retrospective cohort study
Abstract
Objective: To assess the association between long term prescription opioid treatment medically dispensed for non-cancer pain and the initiation of injection drug use (IDU) among individuals without a history of substance use.
Design: Retrospective cohort study.
Setting: Large administrative data source (containing information for about 1.7 million individuals tested for hepatitis C virus or HIV in British Columbia, Canada) with linkage to administrative health databases, including dispensations from community pharmacies.
Participants: Individuals age 11-65 years and without a history of substance use (except alcohol) at baseline.
Main outcome measures: Episodes of prescription opioid use for non-cancer pain were identified based on drugs dispensed between 2000 and 2015. Episodes were classified by the increasing length and intensity of opioid use (acute (lasting <90 episode days), episodic (lasting ≥90 episode days; with <90 days' drug supply and/or <50% episode intensity), and chronic (lasting ≥90 episode days; with ≥90 days' drug supply and ≥50% episode intensity)). People with a chronic episode were matched 1:1:1:1 on socioeconomic variables to those with episodic or acute episodes and to those who were opioid naive. IDU initiation was identified by a validated administrative algorithm with high specificity. Cox models weighted by inverse probability of treatment weights assessed the association between opioid use category (chronic, episodic, acute, opioid naive) and IDU initiation.
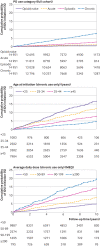
Results: 59 804 participants (14 951 people from each opioid use category) were included in the matched cohort, and followed for a median of 5.8 years. 1149 participants initiated IDU. Cumulative probability of IDU initiation at five years was highest for participants with chronic opioid use (4.0%), followed by those with episodic use (1.3%) and acute use (0.7%), and those who were opioid naive (0.4%). In the inverse probability of treatment weighted Cox model, risk of IDU initiation was 8.4 times higher for those with chronic opioid use versus those who were opioid naive (95% confidence interval 6.4 to 10.9). In a sensitivity analysis limited to individuals with a history of chronic pain, cumulative risk for those with chronic use (3.4% within five years) was lower than the primary results, but the relative risk was not (hazard ratio 9.7 (95% confidence interval 6.5 to 14.5)). IDU initiation was more frequent at higher opioid doses and younger ages.
Conclusions: The rate of IDU initiation among individuals who received chronic prescription opioid treatment for non-cancer pain was infrequent overall (3-4% within five years) but about eight times higher than among opioid naive individuals. These findings could have implications for strategies to prevent IDU initiation, but should not be used as a reason to support involuntary tapering or discontinuation of long term prescription opioid treatment.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from British Columbia Centre for Disease Control and the Canadian Institutes of Health Research for the submitted work; outside of the submitted work, MK has received research grant funding via his institution from Roche Molecular Systems, Siemens Healthcare Diagnostics, and Hologic; MEK has received consulting fees from Biogen; and RCD has performed research and consulted on the over-the-counter product Tylenol for the consumer division of Johnson & Johnson. RCD is executive director of the RADARS System, which performs post-marketing surveillance of prescription drugs. This information is primarily used for regulatory purposes. Subscribers to the RADARS System include the US Food and Drug Administration and some manufacturers of prescription analgesics or treatment for opioid addiction. These companies include Collegium, Purdue Pharma, and Indivior. All other authors report no financial relationships with any organisations that might have an interest in the submitted work in the previous three years. All authors report no other relationships or activities that could appear to have influenced the submitted work.
Figures

Comment in
-
Opioid treatment for non-cancer pain: we need a comprehensive approach.BMJ. 2021 Dec 13;375:n3045. doi: 10.1136/bmj.n3045. BMJ. 2021. PMID: 34903523 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
