Unravelling data for rapid evidence-based response to COVID-19: a summary of the unCoVer protocol
- PMID: 34794999
- PMCID: PMC8602928
- DOI: 10.1136/bmjopen-2021-055630
Unravelling data for rapid evidence-based response to COVID-19: a summary of the unCoVer protocol
Abstract
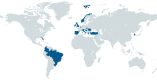
Introduction: unCoVer-Unravelling data for rapid evidence-based response to COVID-19-is a Horizon 2020-funded network of 29 partners from 18 countries capable of collecting and using real-world data (RWD) derived from the response and provision of care to patients with COVID-19 by health systems across Europe and elsewhere. unCoVer aims to exploit the full potential of this information to rapidly address clinical and epidemiological research questions arising from the evolving pandemic.
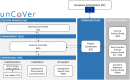
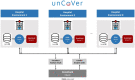
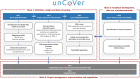
Methods and analysis: From the onset of the COVID-19 pandemic, partners are gathering RWD from electronic health records currently including information from over 22 000 hospitalised patients with COVID-19, and national surveillance and screening data, and registries with over 1 900 000 COVID-19 cases across Europe, with continuous updates. These heterogeneous datasets will be described, harmonised and integrated into a multi-user data repository operated through Opal-DataSHIELD, an interoperable open-source server application. Federated data analyses, without sharing or disclosing any individual-level data, will be performed with the objective to reveal patients' baseline characteristics, biomarkers, determinants of COVID-19 prognosis, safety and effectiveness of treatments, and potential strategies against COVID-19, as well as epidemiological patterns. These analyses will complement evidence from efficacy/safety clinical trials, where vulnerable, more complex/heterogeneous populations and those most at risk of severe COVID-19 are often excluded.
Ethics and dissemination: After strict ethical considerations, databases will be available through a federated data analysis platform that allows processing of available COVID-19 RWD without disclosing identification information to analysts and limiting output to data aggregates. Dissemination of unCoVer's activities will be related to the access and use of dissimilar RWD, as well as the results generated by the pooled analyses. Dissemination will include training and educational activities, scientific publications and conference communications.
Keywords: COVID-19; public health; statistics & research methods.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- World Health Organization . Expanding our understanding of post COVID-19 condition: report of a who webinar, 2021. [Accessed 09 Feb 2021].
-
- World Health Organization (WHO) . COVID-19 clinical management: living guidance, 2021. Available: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
