GOLM1 exacerbates CD8+ T cell suppression in hepatocellular carcinoma by promoting exosomal PD-L1 transport into tumor-associated macrophages
- PMID: 34795203
- PMCID: PMC8602261
- DOI: 10.1038/s41392-021-00784-0
GOLM1 exacerbates CD8+ T cell suppression in hepatocellular carcinoma by promoting exosomal PD-L1 transport into tumor-associated macrophages
Abstract
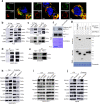
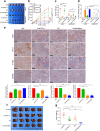
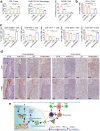
The immunosuppressive microenvironment plays an important role in tumor progression and immunotherapy responses. Golgi membrane protein 1 (GOLM1) is correlated to hepatocellular carcinoma (HCC) progression and metastasis. However, little is known about the role of GOLM1 in regulating the immunosuppressive environment and its impact on immunotherapeutic efficacy in HCC. In this study, GOLM1 was positively correlated with infiltrating tumor-associated macrophages (TAMs) expressed high levels of programmed death-ligand 1 (PD-L1) and CD8+ T cell suppression in HCC tissues. Both gain- and loss-of-function studies determined a close correlation between GOLM1 and immunosuppression. In the mechanism, GOLM1 promoted COP9 signalosome 5-mediated PD-L1 deubiquitination in HCC cells and increased the transport of PD-L1 into exosomes via suppression of Rab27b expression. Furthermore, co-culture with exosomes derived from HCC cells upregulated the expression of PD-L1 on macrophages. Zoledronic acid in combination with anti-PD-L1 therapy reduced PD-L1+ TAMs infiltration and alleviated CD8+ T cell suppression, resulting in tumor growth inhibition in the mouse HCC model. Together, our study unveils a mechanism by which GOLM1 induces CD8+ T cells suppression through promoting PD-L1 stabilization and transporting PD-L1 into TAMs with exosome dependent. Targeting PD-L1+ TAM could be a novel strategy to enhance the efficacy of anti-PD-L1 therapy in HCC.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Villanueva A. Hepatocellular carcinoma. N. Engl. J. Med. 2019;380:1450–1462. - PubMed
-
- Llovet JM, et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021;7:6. - PubMed
-
- Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J. Clin. 2012;62:394–399. - PubMed
-
- Guerra F, Levi Sandri GB. The problem of the most appropriate curative treatment for hepatocellular carcinoma. When to embolize? When to operate? J. Hepatol. 2015;63:280–281. - PubMed
-
- Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

