Modulation of pancreatic cancer cell sensitivity to FOLFIRINOX through microRNA-mediated regulation of DNA damage
- PMID: 34795259
- PMCID: PMC8602334
- DOI: 10.1038/s41467-021-27099-6
Modulation of pancreatic cancer cell sensitivity to FOLFIRINOX through microRNA-mediated regulation of DNA damage
Abstract
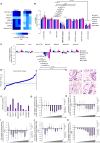
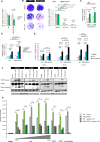
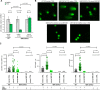
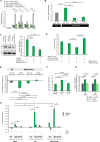
FOLFIRINOX, a combination of chemotherapy drugs (Fluorouracil, Oxaliplatin, Irinotecan -FOI), provides the best clinical benefit in pancreatic ductal adenocarcinoma (PDAC) patients. In this study we explore the role of miRNAs (MIR) as modulators of chemosensitivity to identify potential biomarkers of response. We find that 41 and 84 microRNA inhibitors enhance the sensitivity of Capan1 and MiaPaCa2 PDAC cells respectively. These include a MIR1307-inhibitor that we validate in further PDAC cell lines. Chemotherapy-induced apoptosis and DNA damage accumulation are higher in MIR1307 knock-out (MIR1307KO) versus control PDAC cells, while re-expression of MIR1307 in MIR1307KO cells rescues these effects. We identify binding of MIR1307 to CLIC5 mRNA through covalent ligation of endogenous Argonaute-bound RNAs cross-linking immunoprecipitation assay. We validate these findings in an in vivo model with MIR1307 disruption. In a pilot cohort of PDAC patients undergoing FOLFIRONX chemotherapy, circulating MIR1307 correlates with clinical outcome.
© 2021. The Author(s).
Conflict of interest statement
F.S. has a consultancy role with Bayer; has received research funding from AstraZeneca, Bayer, BMS, Roche; and travel grants from Bayer and Lilly. N.S. has received research funding from AstraZeneca, BMS, Pfizer; honoraria from Eli Lilly, Merck Serono, MSD Oncology, Pierre Fabre, Servier; travel/accommodation from AstraZeneca, BMS, Eli Lilly, Merck, Roche; and has had advisory roles with Pfizer, AstraZeneca, Servier. D.K.C. received research funding from Celgene, AstraZeneca, MSD and Immodulon, and honoraria from Celgene. D.C. has received funding from Amgen, Sanofi, Merrimack, AstraZeneca, Celgene, MedImmune, Bayer, 4SC, Clovis, Eli Lilly, Janssen, Merck; and has an advisory role with OVIBIO. M.F. has received grants from Astellas Pharma and QED Therapeutics; and has advisory roles with Astellas Pharma, Tesaro, and Diaceutics. F.S. received funding from Amal Therapeutics, Bayer, BMS, Dragonfly Therapeutics, Amgen (inst), AstraZeneca (Inst), Bayer (Inst), BMS (Inst), Roche (Inst), Sanofi (Inst), Bayer, Lilly and is Co-Chair EORTC Colon Cancer Task Force. N.V. received honoraria or travel accommodation from Merck Serono, Pfizer, Bayer, Eli-Lilly, and Menarini Silicon Biosystems. P.W. advises and received grants from Astex Pharmaceuticals; advises and owns stock in Nextechinvest; is employed and owns stock in Storm Therapeutics; advises CV6 Therapeutics, Black Diamond Therapeutics, and Vividion Therapeutics; received grants from Vernalis and Merck, and owns stock in Chroma Therapeutics. C.B. received honoraria from Incyte. All other authors declare no competing interests.
Figures



References
-
- Burris HA, 3rd, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 1997;15:2403–2413. - PubMed
-
- Cunningham D, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J. Clin. Oncol. 2009;27:5513–5518. - PubMed
-
- Conroy T, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364:1817–1825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

