Remote diagnosis of surgical-site infection using a mobile digital intervention: a randomised controlled trial in emergency surgery patients
- PMID: 34795398
- PMCID: PMC8602321
- DOI: 10.1038/s41746-021-00526-0
Remote diagnosis of surgical-site infection using a mobile digital intervention: a randomised controlled trial in emergency surgery patients
Abstract
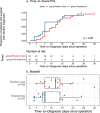
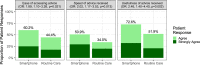
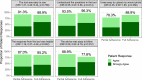
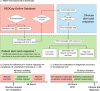
Surgical site infections (SSI) cause substantial morbidity and pose a burden to acute healthcare services after surgery. We aimed to investigate whether a smartphone-delivered wound assessment tool can expedite diagnosis and treatment of SSI after emergency abdominal surgery. This single-blinded randomised control trial (NCT02704897) enroled adult emergency abdominal surgery patients in two tertiary care hospitals. Patients were randomised (1:1) to routine postoperative care or additional access to a smartphone-delivered wound assessment tool for 30-days postoperatively. Patient-reported SSI symptoms and wound photographs were requested on postoperative days 3, 7, and 15. The primary outcome was time-to-diagnosis of SSI (Centers for Disease Control definition). 492 patients were randomised (smartphone intervention: 223; routine care: 269). There was no significant difference in the 30-day SSI rate between trial arms: 21 (9.4%) in smartphone vs 20 (7.4%, p = 0.513) in routine care. Among the smartphone group, 32.3% (n = 72) did not utilise the tool. There was no significant difference in time-to-diagnosis of SSI for patients receiving the intervention (-2.5 days, 95% CI: -6.6-1.6, p = 0.225). However, patients in the smartphone group had 3.7-times higher odds of diagnosis within 7 postoperative days (95% CI: 1.02-13.51, p = 0.043). The smartphone group had significantly reduced community care attendance (OR: 0.57, 95% CI: 0.34-0.94, p = 0.030), similar hospital attendance (OR: 0.76, 95% CI: 0.28-1.96, p = 0.577), and significantly better experiences in accessing care (OR: 2.02, 95% CI: 1.17-3.53, p = 0.013). Smartphone-delivered wound follow-up is feasible following emergency abdominal surgery. This can facilitate triage to the appropriate level of assessment required, allowing earlier postoperative diagnosis of SSI.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Centre for Disease Control and Prevention (CDC) National Healthcare Safety Network (NHSN). Patient Safety Component (PSC) Manual Chapter 9: Surgical site infection (SSI) event. (CDC, Atlanta, 2016).
-
- Office of Communications (OFCOM). Communications Market Report 2018. (OFCOM, London, 2019).
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

