Characterising 18F-fluciclovine uptake in breast cancer through the use of dynamic PET/CT imaging
- PMID: 34795409
- PMCID: PMC8854436
- DOI: 10.1038/s41416-021-01623-3
Characterising 18F-fluciclovine uptake in breast cancer through the use of dynamic PET/CT imaging
Abstract
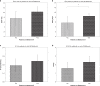
Background: 18F-fluciclovine is a synthetic amino acid positron emission tomography (PET) radiotracer that is approved for use in prostate cancer. In this clinical study, we characterised the kinetic model best describing the uptake of 18F-fluciclovine in breast cancer and assessed differences in tracer kinetics and static parameters for different breast cancer receptor subtypes and tumour grades.
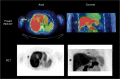
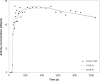
Methods: Thirty-nine patients with pathologically proven breast cancer underwent 20-min dynamic PET/computed tomography imaging following the administration of 18F-fluciclovine. Uptake into primary breast tumours was evaluated using one- and two-tissue reversible compartmental kinetic models and static parameters.
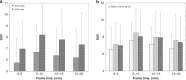
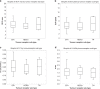
Results: A reversible one-tissue compartment model was shown to best describe tracer uptake in breast cancer. No significant differences were seen in kinetic or static parameters for different tumour receptor subtypes or grades. Kinetic and static parameters showed a good correlation.
Conclusions: 18F-fluciclovine has potential in the imaging of primary breast cancer, but kinetic analysis may not have additional value over static measures of tracer uptake.
Clinical trial registration: NCT03036943.
© 2021. The Author(s).
Conflict of interest statement
At the time of submission, EJT is an employee of Blue Earth Diagnostics. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, HEE or the Department of Health and Social Care. The authors declare no competing interests.
Figures

References
-
- Mcconathy J. 18F-Fluciclovine (FACBC) and its potential use for breast cancer imaging. J Nucl Med. 2016;57:1329–30. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

