Characterisation of a novel KRAS G12C inhibitor ASP2453 that shows potent anti-tumour activity in KRAS G12C-mutated preclinical models
- PMID: 34795410
- PMCID: PMC8888662
- DOI: 10.1038/s41416-021-01629-x
Characterisation of a novel KRAS G12C inhibitor ASP2453 that shows potent anti-tumour activity in KRAS G12C-mutated preclinical models
Abstract
Background: KRAS is one of the most frequently mutated oncogenes in various cancers, and several novel KRAS G12C direct inhibitors are now in clinical trials. Here, we characterised the anti-tumour efficacy of ASP2453, a novel KRAS G12C inhibitor, in preclinical models of KRAS G12C-mutated cancer.
Methods: We evaluated the in vitro and in vivo activity of ASP2453, alone or in combination with targeted agents and immune checkpoint inhibitors, in KRAS G12C-mutated cancer cells and xenograft models. We also assessed pharmacological differences between ASP2453 and AMG 510, another KRAS G12C inhibitor, using an SPR assay, washout experiments and an AMG 510-resistant xenograft model.
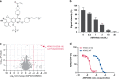
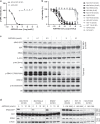
Results: ASP2453 potently and selectively inhibited KRAS G12C-mediated growth, KRAS activation and downstream signalling in vitro and in vivo, and improved the anti-tumour effects of targeted agents and immune checkpoint inhibitors. Further, ASP2453 had more rapid binding kinetics to KRAS G12C protein and showed more potent inhibitory effects on KRAS activation and cell proliferation after washout than AMG 510. ASP2453 also induced tumour regression in an AMG 510-resistant xenograft model.
Conclusions: ASP2453 is a potential therapeutic agent for KRAS G12C-mutated cancer. ASP2453 showed efficacy in AMG 510-resistant tumours, even among compounds with the same mode of action.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
All authors are employees of Astellas Pharma Inc. and its affiliates.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

