Photobiomodulation Promotes Repair Following Spinal Cord Injury by Regulating the Transformation of A1/A2 Reactive Astrocytes
- PMID: 34795557
- PMCID: PMC8593167
- DOI: 10.3389/fnins.2021.768262
Photobiomodulation Promotes Repair Following Spinal Cord Injury by Regulating the Transformation of A1/A2 Reactive Astrocytes
Abstract
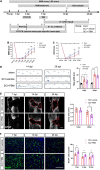
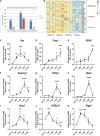
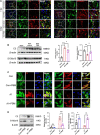
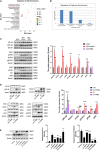
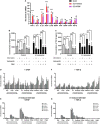
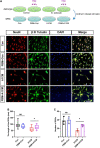
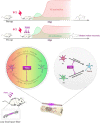
After spinal cord injury (SCI), reactive astrocytes can be classified into two distinctive phenotypes according to their different functions: neurotoxic (A1) astrocytes and neuroprotective (A2) astrocytes. Our previous studies proved that photobiomodulation (PBM) can promote motor function recovery and improve tissue repair after SCI, but little is known about the underlying mechanism. Therefore, we aimed to investigate whether PBM contributes to repair after SCI by regulating the activation of astrocytes. Male rats subjected to clip-compression SCI were treated with PBM for two consecutive weeks, and the results showed that recovery of motor function was improved, the lesion cavity size was reduced, and the number of neurons retained was increased. We determined the time course of A1/A2 astrocyte activation after SCI by RNA sequencing (RNA-Seq) and verified that PBM inhibited A1 astrocyte activation and promoted A2 astrocyte activation at 7 days postinjury (dpi) and 14 dpi. Subsequently, potential signaling pathways related to A1/A2 astrocyte activation were identified by GO function analysis and KEGG pathway analysis and then studied in animal experiments and preliminarily analyzed in cultured astrocytes. Next, we observed that the expression of basic fibroblast growth factor (bFGF) and transforming growth factor-β (TGF-β) was upregulated by PBM and that both factors contributed to the transformation of A1/A2 astrocytes in a dose-dependent manner. Finally, we found that PBM reduced the neurotoxicity of A1 astrocytes to dorsal root ganglion (DRG) neurons. In conclusion, PBM can promote better recovery after SCI, which may be related to the transformation of A1/A2 reactive astrocytes.
Keywords: A1/A2 astrocytes; basic fibroblast growth factor; photobiomodulation; spinal cord injury; transforming growth factor-β.
Copyright © 2021 Wang, Zhang, Zhu, Liang, Zuo, Ju, Song, Li, Hu and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Photobiomodulation inhibits the activation of neurotoxic microglia and astrocytes by inhibiting Lcn2/JAK2-STAT3 crosstalk after spinal cord injury in male rats.J Neuroinflammation. 2021 Nov 5;18(1):256. doi: 10.1186/s12974-021-02312-x. J Neuroinflammation. 2021. PMID: 34740378 Free PMC article.
-
Photobiomodulation Attenuates Neurotoxic Polarization of Macrophages by Inhibiting the Notch1-HIF-1α/NF-κB Signalling Pathway in Mice With Spinal Cord Injury.Front Immunol. 2022 Mar 17;13:816952. doi: 10.3389/fimmu.2022.816952. eCollection 2022. Front Immunol. 2022. PMID: 35371065 Free PMC article.
-
Astrocyte-conditional knockout of MOB2 inhibits the phenotypic conversion of reactive astrocytes from A1 to A2 following spinal cord injury in mice.Int J Biol Macromol. 2025 Apr;300:140289. doi: 10.1016/j.ijbiomac.2025.140289. Epub 2025 Jan 23. Int J Biol Macromol. 2025. PMID: 39863205
-
Inhibition of A1 Astrocytes and Activation of A2 Astrocytes for the Treatment of Spinal Cord Injury.Neurochem Res. 2023 Mar;48(3):767-780. doi: 10.1007/s11064-022-03820-9. Epub 2022 Nov 23. Neurochem Res. 2023. PMID: 36418652 Review.
-
Targeting astrocytes polarization after spinal cord injury: a promising direction.Front Cell Neurosci. 2024 Oct 16;18:1478741. doi: 10.3389/fncel.2024.1478741. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39479524 Free PMC article. Review.
Cited by
-
The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: Mechanisms and therapeutic opportunities.Cell Prolif. 2022 Sep;55(9):e13275. doi: 10.1111/cpr.13275. Epub 2022 Jun 26. Cell Prolif. 2022. PMID: 35754255 Free PMC article. Review.
-
Photobiomodulation augments the effects of mitochondrial transplantation in the treatment of spinal cord injury in rats by facilitating mitochondrial transfer to neurons via Connexin 36.Bioeng Transl Med. 2022 Dec 21;8(3):e10473. doi: 10.1002/btm2.10473. eCollection 2023 May. Bioeng Transl Med. 2022. PMID: 37206245 Free PMC article.
-
Ginsenoside Rg1 promotes astrocyte-to-neuron transdifferentiation in rat and its possible mechanism.CNS Neurosci Ther. 2023 Jan;29(1):256-269. doi: 10.1111/cns.14000. Epub 2022 Nov 9. CNS Neurosci Ther. 2023. PMID: 36352836 Free PMC article.
-
The role of immune cells and associated immunological factors in the immune response to spinal cord injury.Front Immunol. 2023 Jan 5;13:1070540. doi: 10.3389/fimmu.2022.1070540. eCollection 2022. Front Immunol. 2023. PMID: 36685599 Free PMC article. Review.
-
Implantable and transcutaneous photobiomodulation promote neuroregeneration and recovery of lost function after spinal cord injury.Bioeng Transl Med. 2024 Apr 25;9(6):e10674. doi: 10.1002/btm2.10674. eCollection 2024 Nov. Bioeng Transl Med. 2024. PMID: 39545078 Free PMC article.
References
LinkOut - more resources
Full Text Sources

