Role of Cleaved PINK1 in Neuronal Development, Synaptogenesis, and Plasticity: Implications for Parkinson's Disease
- PMID: 34795558
- PMCID: PMC8593325
- DOI: 10.3389/fnins.2021.769331
Role of Cleaved PINK1 in Neuronal Development, Synaptogenesis, and Plasticity: Implications for Parkinson's Disease
Abstract
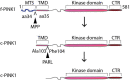
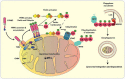
Mitochondrial dysfunction plays a significant role in the pathogenesis of Parkinson's disease (PD). Consistent with this concept, loss of function mutations in the serine/threonine kinase- PINK1 (PTEN-induced putative kinase-1) causes autosomal recessive early onset PD. While the functional role of f-PINK1 (full-length PINK1) in clearing dysfunctional mitochondria via mitophagy is extensively documented, our understanding of specific physiological roles that the non-mitochondrial pool of PINK1 imparts in neurons is more limited. PINK1 is proteolytically processed in the intermembrane space and matrix of the mitochondria into functional cleaved products (c-PINK1) that are exported to the cytosol. While it is clear that posttranslational processing of PINK1 depends on the mitochondria's oxidative state and structural integrity, the functional roles of c-PINK1 in modulating neuronal functions are poorly understood. Here, we review the diverse roles played by c-PINK1 in modulating various neuronal functions. Specifically, we describe the non-canonical functional roles of PINK1, including but not limited to: governing mitochondrial movement, neuronal development, neuronal survival, and neurogenesis. We have published that c-PINK1 stimulates neuronal plasticity and differentiation via the PINK1-PKA-BDNF signaling cascade. In addition, we provide insight into how mitochondrial membrane potential-dependent processing of PINK1 confers conditional retrograde signaling functions to PINK1. Further studies delineating the role of c-PINK1 in neurons would increase our understanding regarding the role played by PINK1 in PD pathogenesis.
Keywords: BDNF (brain derived neurotrophic factor); PKA signaling; Parkinson’s disease; cleaved PINK1; mitochondrial retrograde signaling; neuronal plasticity and neurogenesis.
Copyright © 2021 Soman and Dagda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

